Question: Unknown #:.36............. Initial Burette Reading (mL) Final Burette Reading (mL) Volume of KMnO4 used (L) #moles KMnO4 used #moles of oxalate in diluted solution
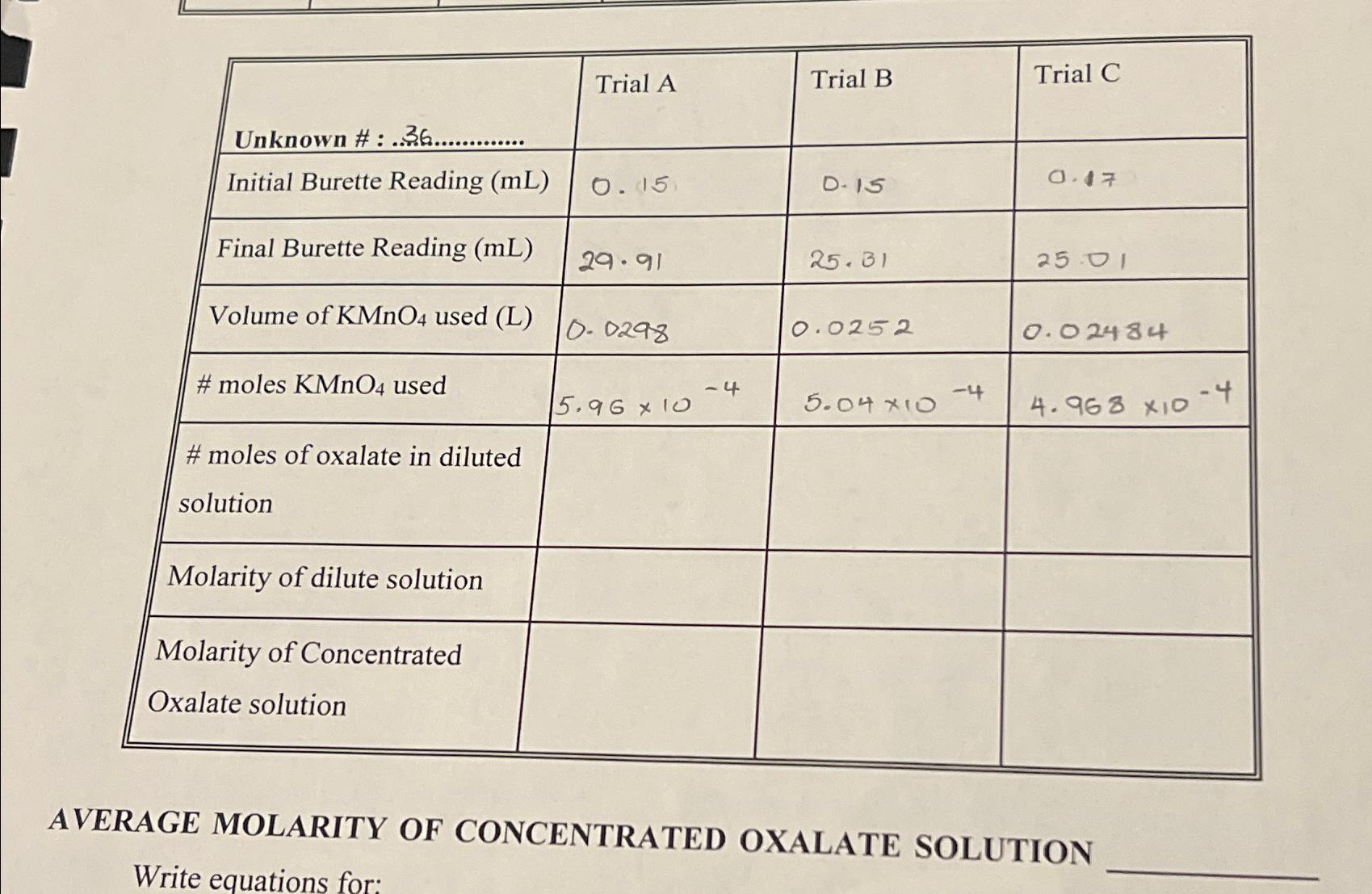
Unknown #:.36............. Initial Burette Reading (mL) Final Burette Reading (mL) Volume of KMnO4 used (L) #moles KMnO4 used #moles of oxalate in diluted solution Molarity of dilute solution Molarity of Concentrated Oxalate solution Trial A 0.15 29.91 0.0298 5.96 x 10 -4 Trial B D-15 25.31 0.0252 5.04 *10 -4 Trial C 0.47 25.01 0.02484 4.968 X10 AVERAGE MOLARITY OF CONCENTRATED OXALATE SOLUTION Write equations for: -4
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
Balanced ionic reaction betweeen oxalate and KMnO4 is VII 2 5 C0 2 MnO 16H moles of KMnO ... View full answer

Get step-by-step solutions from verified subject matter experts


