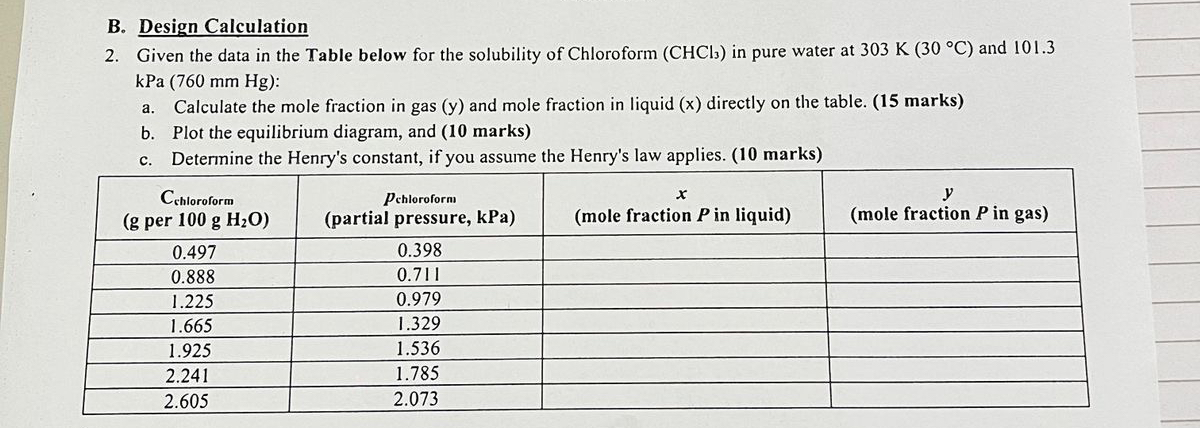
Question: URGNT PLEASE ANSWER NOWW B . Design Calculation 2 . Given the data in the Table below for the solubility of Chloroform ( C H
URGNT PLEASE ANSWER NOWW
B Design Calculation
Given the data in the Table below for the solubility of Chloroform in pure water at and kPa:
a Calculate the mole fraction in gas and mole fraction in liquid directly on the table. marks
b Plot the equilibrium diagram, and marks
c Determine the Henry's constant, if you assume the Henry's law applies. marks
tabletable
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


