Question: ursel_21841 Question 6 1 Point On analysis, an equilibrium mixture for the reaction 2H2(g) 2H2(g) + S2(g) was found to contain 1.0 mol H2S, 16.0
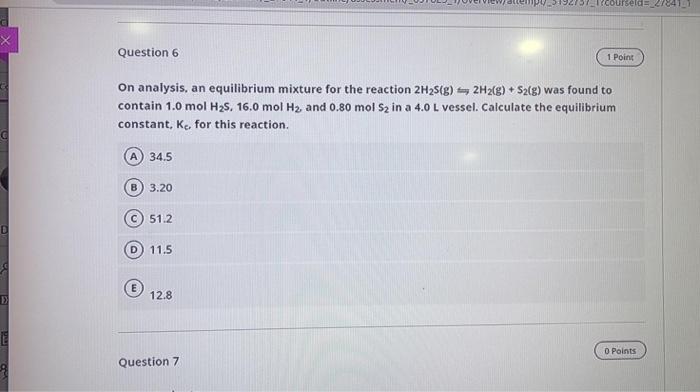
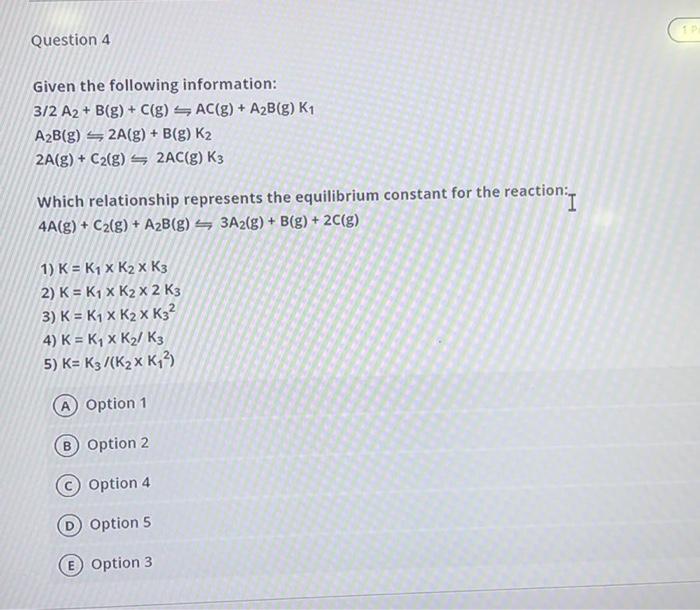
ursel_21841 Question 6 1 Point On analysis, an equilibrium mixture for the reaction 2H2(g) 2H2(g) + S2(g) was found to contain 1.0 mol H2S, 16.0 mol H2, and 0.80 mol Sz in a 4.0 L vessel. Calculate the equilibrium constant, K, for this reaction. A 34.5 B 3.20 51.2 D) 11.5 6 E 12.8 0 Points Question 7 Question 4 + Given the following information: 3/2 A2+ B(g) + C(g) SAC(g) + A2B(g) K1 A2B(g) 2A(g) + B(g) K2 2A(g) + C2(g) = 2AC(g) K3 Which relationship represents the equilibrium constant for the reaction:- action. I 4A(g) + C2(g) + A2B(g) 3A2(g) + B(g) + 2C(g) 1) K = K1 x K2 x K3 2) K = K1 x K2 x 2 K3 3) K = K1 x K2 X K32 4) K = Ky x K / K3 5) K= K3/(K2x K43) Option 1 B Option 2 C Option 4 D Option 5 E Option 3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


