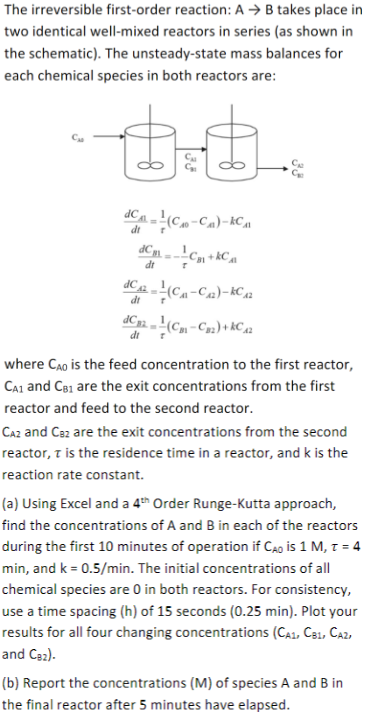
Question: USE EXCEL: The irreversible first - order reaction: A B takes place in two identical well - mixed reactors in series ( as shown in
USE EXCEL: The irreversible firstorder reaction: takes place in
two identical wellmixed reactors in series as shown in
the schematic The unsteadystate mass balances for
each chemical species in both reactors are:
where is the feed concentration to the first reactor,
and are the exit concentrations from the first
reactor and feed to the second reactor.
and are the exit concentrations from the second
reactor, is the residence time in a reactor, and is the
reaction rate constant.
a Using Excel and a Order RungeKutta approach,
find the concentrations of A and B in each of the reactors
during the first minutes of operation if is
min, and The initial concentrations of all
chemical species are in both reactors. For consistency,
use a time spacing of seconds min Plot your
results for all four changing concentrations
and
b Report the concentrations M of species A and B in
the final reactor after minutes have elapsed.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


