Question: Use Matlab PLs QUESTION 2 Upload your published pdf file for problem #2 here ... if you are not able to successfully publish a file,
 Use Matlab PLs
Use Matlab PLs
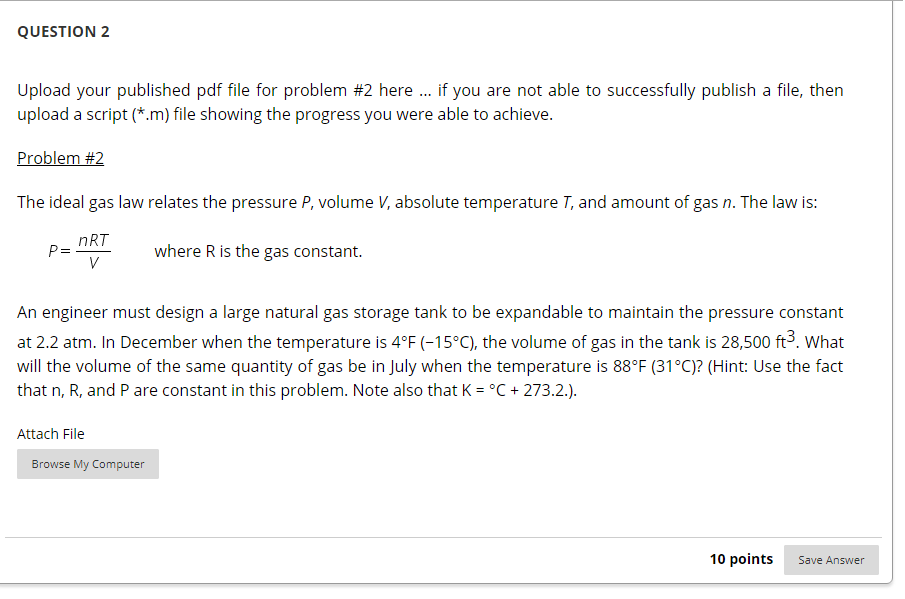
QUESTION 2 Upload your published pdf file for problem #2 here ... if you are not able to successfully publish a file, then upload a script (*.m) file showing the progress you were able to achieve. Problem #2 The ideal gas law relates the pressure P, volume V, absolute temperature T, and amount of gas n. The law is: P= nRT - where R is the gas constant. An engineer must design a large natural gas storage tank to be expandable to maintain the pressure constant at 2.2 atm. In December when the temperature is 4F (-15C), the volume of gas in the tank is 28,500 ft. What will the volume of the same quantity of gas be in July when the temperature is 88F (31C)? (Hint: Use the fact that n, R, and P are constant in this problem. Note also that K = C + 273.2.). Attach File Browse My Computer 10 points Save
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


