Question: Use Microsoft Excel, screenshot your work, and please show the formula you used in excel in solving the problem below: The reaction of calcination is
Use Microsoft Excel, screenshot your work, and please show the formula you used in excel in solving the problem below: 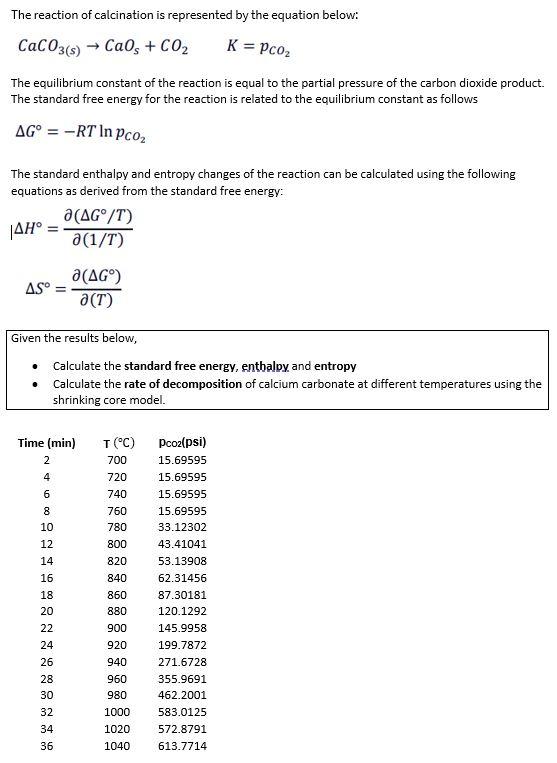
The reaction of calcination is represented by the equation below: CaCO3(s) CaOs +CO2 K = Pc0 The equilibrium constant of the reaction is equal to the partial pressure of the carbon dioxide product. The standard free energy for the reaction is related to the equilibrium constant as follows AG = -RT In pco The standard enthalpy and entropy changes of the reaction can be calculated using the following equations as derived from the standard free energy: a(AG/T) |AH a(1/T) a(AG) AS = a (T) Given the results below, Calculate the standard free energy, enthalpy and entropy Calculate the rate of decomposition of calcium carbonate at different temperatures using the shrinking core model. Time (min) T (C) Pco2(psi) 15.69595 700 720 15.69595 740 15.69595 760 15.69595 780 33.12302 800 43.41041 820 53.13908 840 62.31456 860 87.30181 880 120.1292 900 145.9958 920 199.7872 940 271.6728 960 355.9691 980 462.2001 1000 583.0125 1020 572.8791 1040 613.7714 = 24600 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


