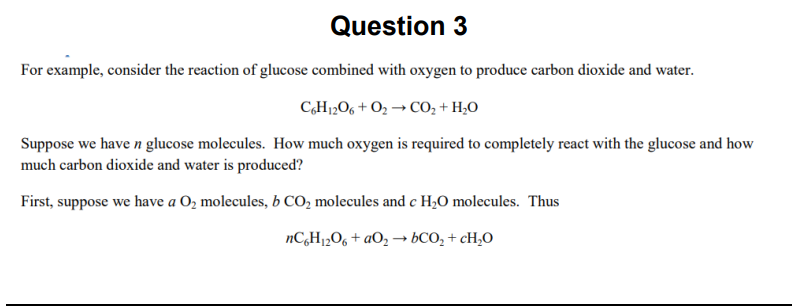
Question: Use octave to solve this problem. Question 3 For example, consider the reaction of glucose combined with oxygen to produce carbon dioxide and water. CH
 Use octave to solve this problem.
Use octave to solve this problem.
Question 3 For example, consider the reaction of glucose combined with oxygen to produce carbon dioxide and water. CH 206+02 CO2 + H20 Suppose we have n glucose molecules. How much oxygen is required to completely react with the glucose and how much carbon dioxide and water is produced? First, suppose we have a 02 molecules, b CO2 molecules and c H2O molecules. Thus nCH 206+a026CO2 + cH,0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


