Question: Use python to solve the below The van der Waals equation of state can be used to calculate the compressibility factors (Z) of gases. One
Use python to solve the below
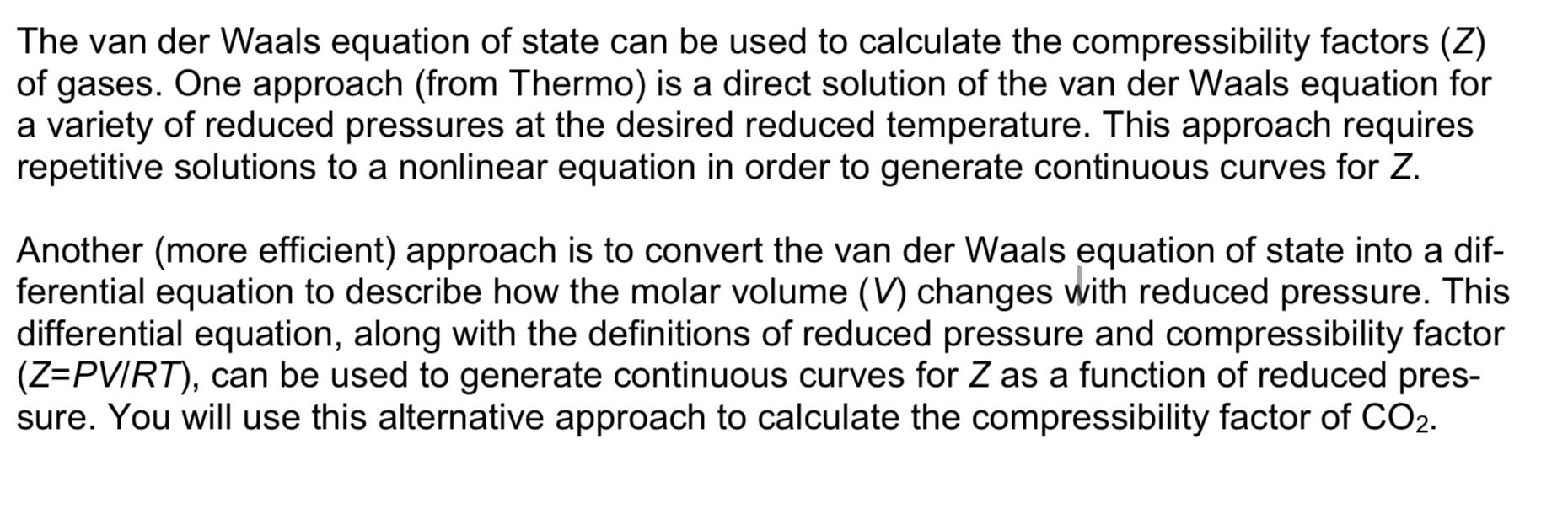
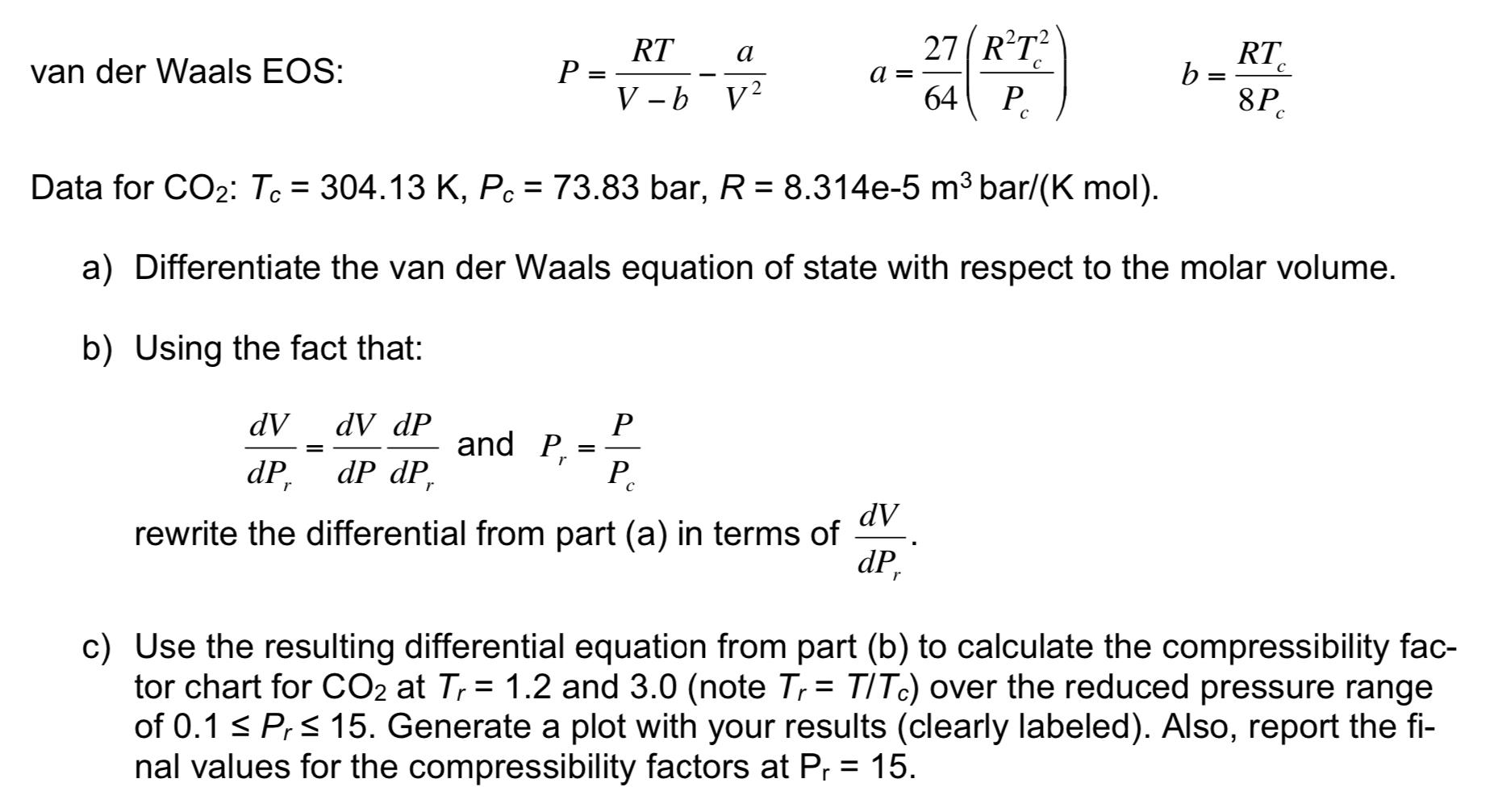
The van der Waals equation of state can be used to calculate the compressibility factors (Z) of gases. One approach (from Thermo) is a direct solution of the van der Waals equation for a variety of reduced pressures at the desired reduced temperature. This approach requires repetitive solutions to a nonlinear equation in order to generate continuous curves for Z. Another (more efficient) approach is to convert the van der Waals equation of state into a differential equation to describe how the molar volume (V) changes with reduced pressure. This differential equation, along with the definitions of reduced pressure and compressibility factor ( Z=PVIRT), can be used to generate continuous curves for Z as a function of reduced pressure. You will use this alternative approach to calculate the compressibility factor of CO2. van der Waals EOS: P=VbRTV2aa=6427(PcR2Tc2) b=8PcRTc Data for CO2:Tc=304.13K,Pc=73.83bar,R=8.314e5m3bar/(Kmol). a) Differentiate the van der Waals equation of state with respect to the molar volume. b) Using the fact that: dPrdV=dPdVdPrdPandPr=PcP rewrite the differential from part (a) in terms of dPrdV. c) Use the resulting differential equation from part (b) to calculate the compressibility factor chart for CO2 at Tr=1.2 and 3.0 (note Tr=T/Tc ) over the reduced pressure range of 0.1Pr15. Generate a plot with your results (clearly labeled). Also, report the final values for the compressibility factors at Pr=15
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


