Question: Use the Aeferences to accest important values if needed for this question. How many grams of Cu(OH) 2 will precipitate when excess KOH solution is
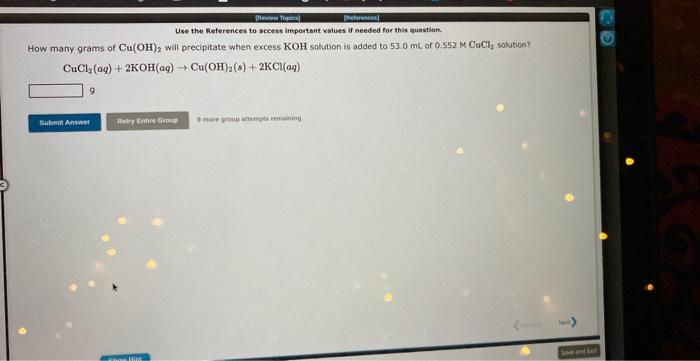
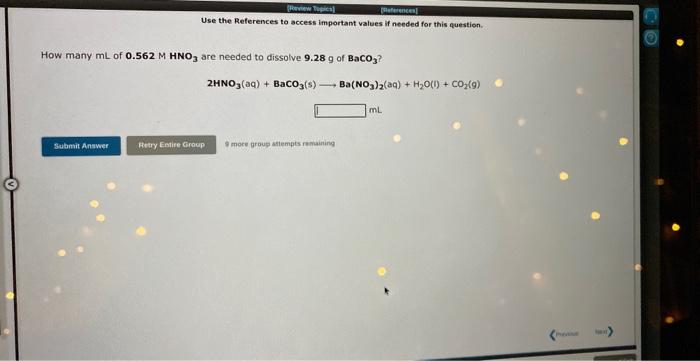
Use the Aeferences to accest important values if needed for this question. How many grams of Cu(OH) 2 will precipitate when excess KOH solution is added to 53.0mL of 0.552M CuCl, solution? CuCl2(aq)+2KOH(aq)Cu(OH)2(s)+2KCl(aq) 9 Use the References to access important values if needed for this question. are needed to dissolve 9.28g of BaCO3 ? 2HNO3(aq)+BaCO3(s)Ba(NO3)2(aq)+H2O(1)+CO2(g) i moce jroug atempts r mainirs
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


