Question: Use the References to access important values if needed for this question. How many grams of PbBr2 will precipitate when excess CrBr3 solution is added
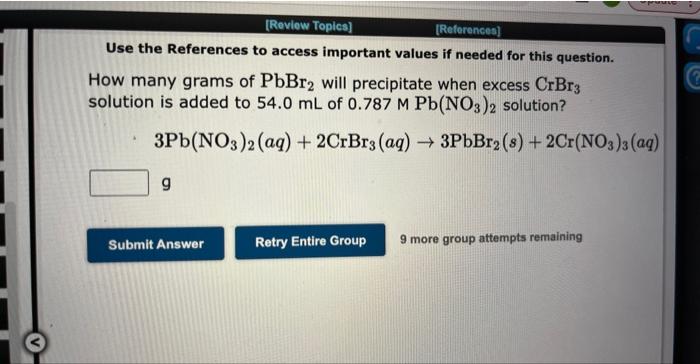
Use the References to access important values if needed for this question. How many grams of PbBr2 will precipitate when excess CrBr3 solution is added to 54.0mL of 0.787MPb(NO3)2 solution? 3Pb(NO3)2(aq)+2CrBr3(aq)3PbBr2(s)+2Cr(NO3)3(aq) g 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


