Question: Use the conservation of mass interactive to answer the question. Consider the reaction. Na2S(s)+2HCl(aq)2NaCl(aq)+H2S(g) Select the true statements. The total number of hydrogen atoms before
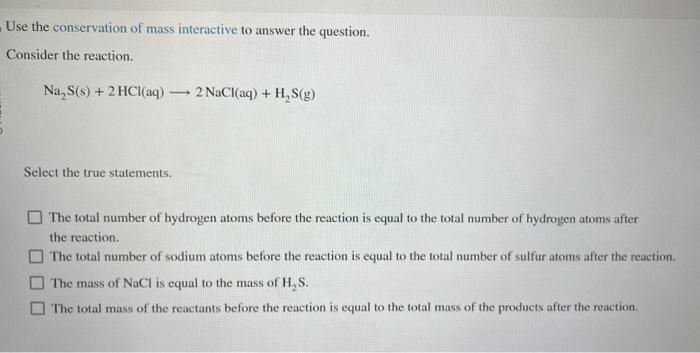
Use the conservation of mass interactive to answer the question. Consider the reaction. Na2S(s)+2HCl(aq)2NaCl(aq)+H2S(g) Select the true statements. The total number of hydrogen atoms before the reaction is equal to the total number of hydrogen atoms after the reaction. The total number of sodium atoms before the reaction is equal to the total number of sulfur atoms after the reaction. The mass of NaCl is equal to the mass of H2S. The total mass of the reactants before the reaction is equal to the total mass of the products after the reaction
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


