Question: Use the diffraction graphs to solve. X-ray process Auger process AES Fermi level M45 0 3.7 eV WA M23 34.6 eV h 364 354 451
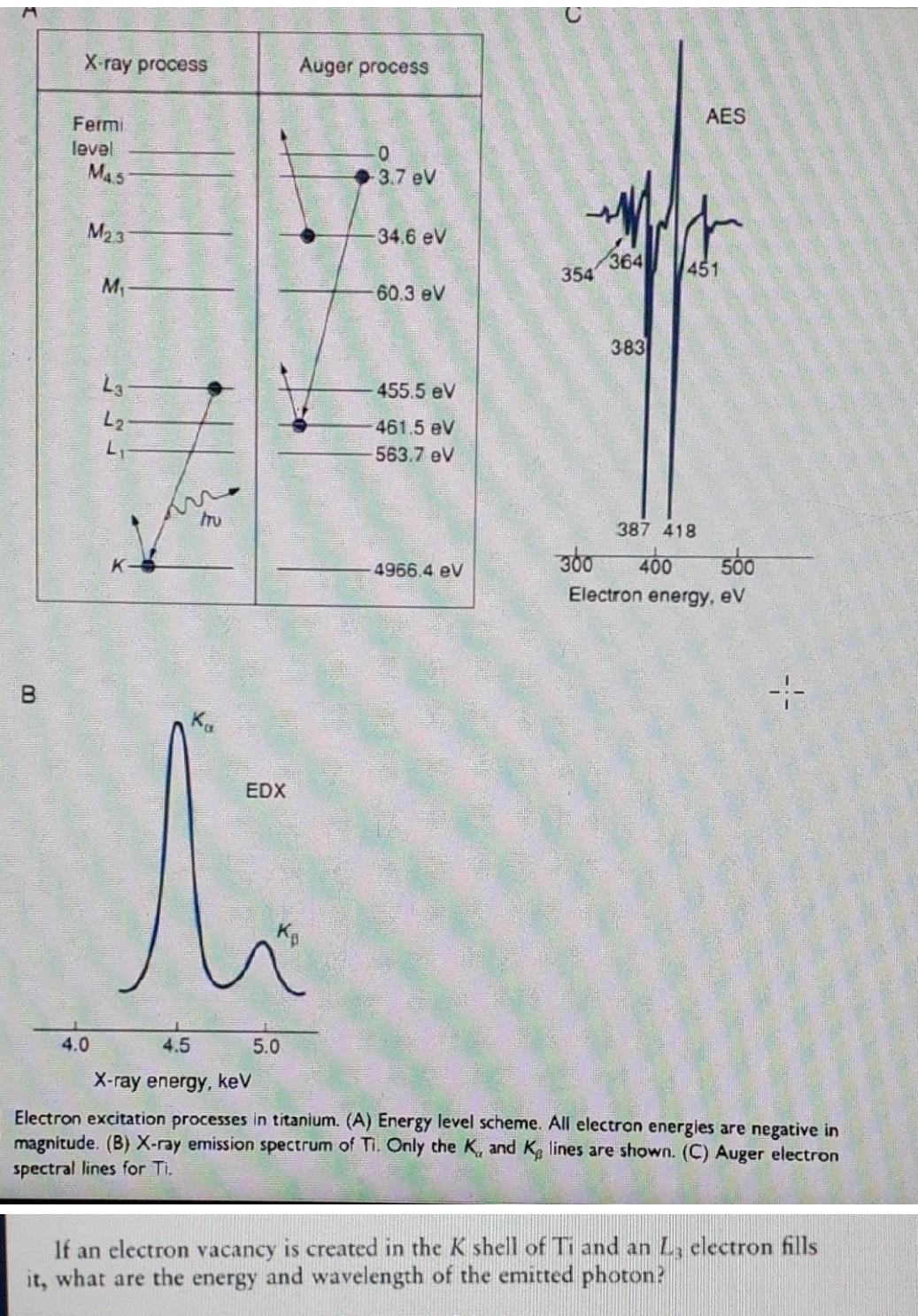
Use the diffraction graphs to solve.
X-ray process Auger process AES Fermi level M45 0 3.7 eV WA M23 34.6 eV h 364 354 451 M 60.3 eV 383 455.5 eV L3 L2 L 461.5 eV -563.7 eV tru 387 418 360 400 Electron energy, eV 500 4966.4 eV B -- EDX 4.0 4.5 5.0 X-ray energy, keV Electron excitation processes in titanium. (A) Energy level scheme. All electron energies are negative in magnitude. (B) X-ray emission spectrum of Ti. Only the K, and Kp lines are shown. (C) Auger electron spectral lines for Ti. If an electron vacancy is created in the K shell of Ti and an electron fills it, what are the energy and wavelength of the emitted photon
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


