Question: Use the exact values you enter to make later calculations. Consider the following figure. Consider a Pb-15%. Sn alloy. During solidification, determine the following. (For
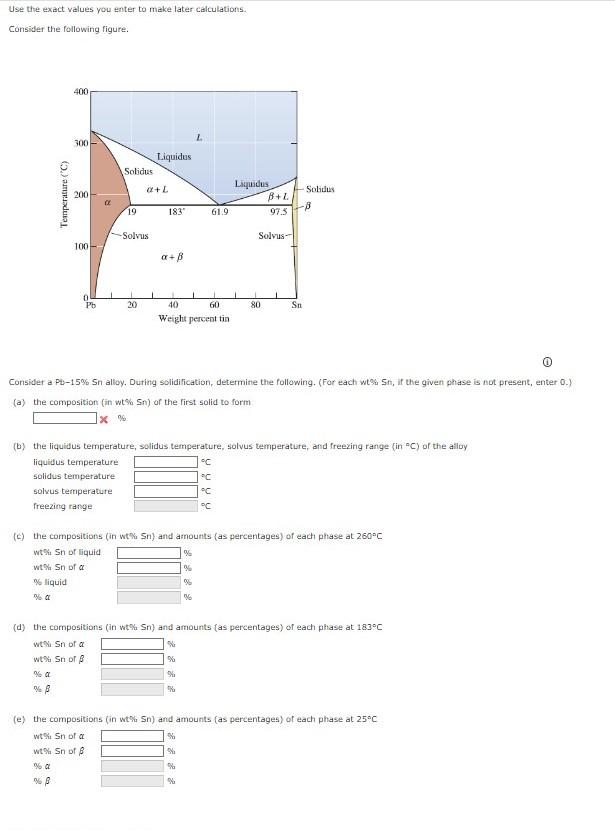
Use the exact values you enter to make later calculations. Consider the following figure. Consider a Pb-15\%. Sn alloy. During solidification, determine the following. (For each wt\%u St, if the given phase is not present, enter 0.) (a) the composition (in wt\% Sn ) af the first solid to form x6 (b) the liquidus temperature, solidus temperature, solvus temperature, and freezing range (in " C ) of the alloy liquidustemperaturesolidustemperaturesolvustemperaturefreezingrangeCCCC (c) the compositions (in wtw 5n ) and amounts (as percentages) of each phase at 260C wt%Snofliquidwt%Snof%sliquid%aof%%6%6 (d) the compositions (in wt\% 5n ) and amounts (as percentages) of each phase at 183C wt?Snofwt%Snofoo%%%% (e) the compositions (in wt\% 5n ) and amounts (as percentages) of each phase at 25C wtris sin of a wt Sn of % %
Step by Step Solution
There are 3 Steps involved in it
Based on the phase diagram provided in the image and the alloy composition of Pb15 wt Sn we can now calculate all the required data stepbystep a Compo... View full answer

Get step-by-step solutions from verified subject matter experts


