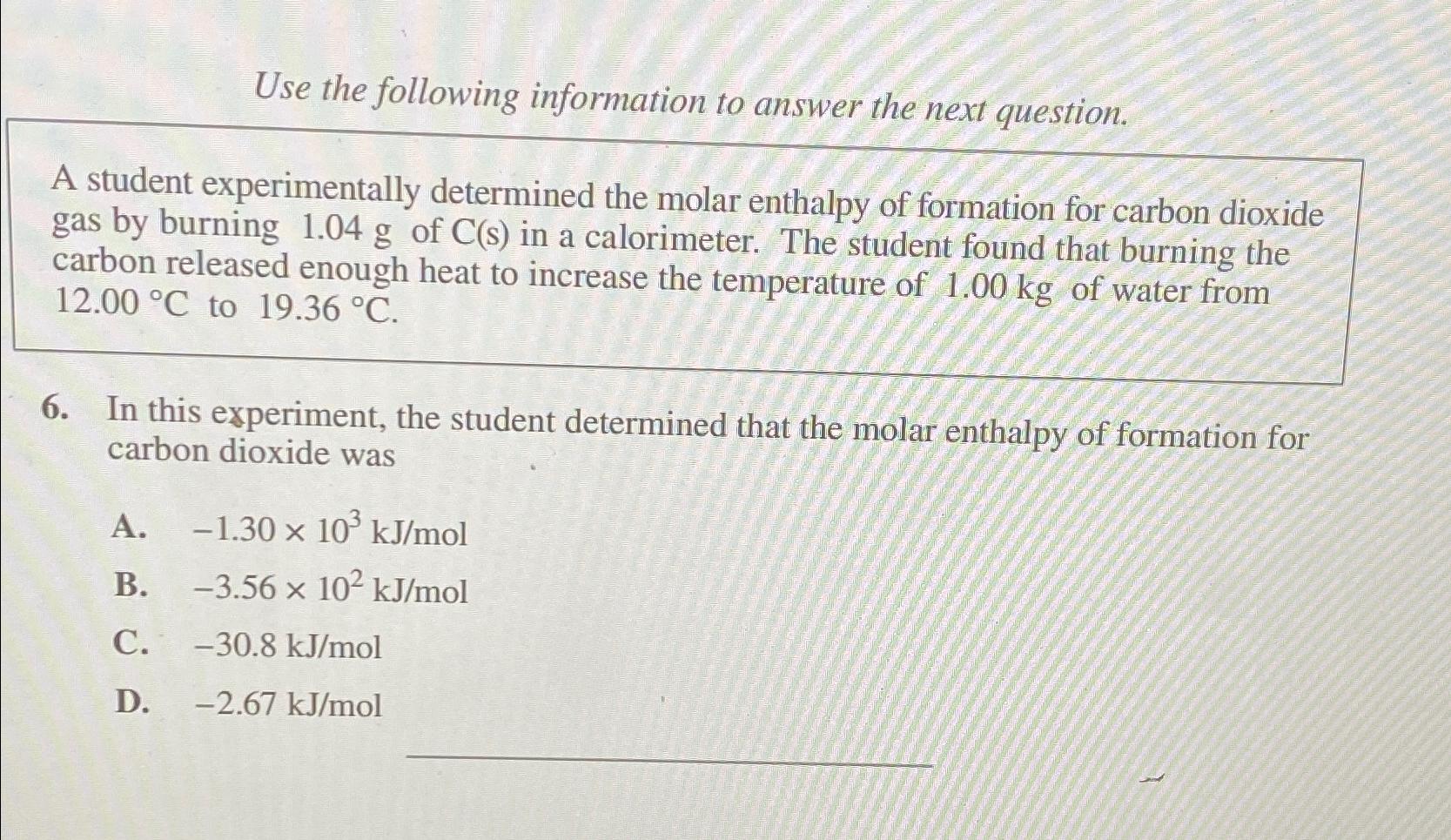
Question: Use the following information to answer the next question. A student experimentally determined the molar enthalpy of formation for carbon dioxide gas by burning 1
Use the following information to answer the next question.
A student experimentally determined the molar enthalpy of formation for carbon dioxide gas by burning of in a calorimeter. The student found that burning the carbon released enough heat to increase the temperature of of water from to
In this experiment, the student determined that the molar enthalpy of formation for carbon dioxide was
A
B
C
D
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


