Question: Use the following information to answer the next question A group of students performed a titration and obtained the following titration curve for an acid-base
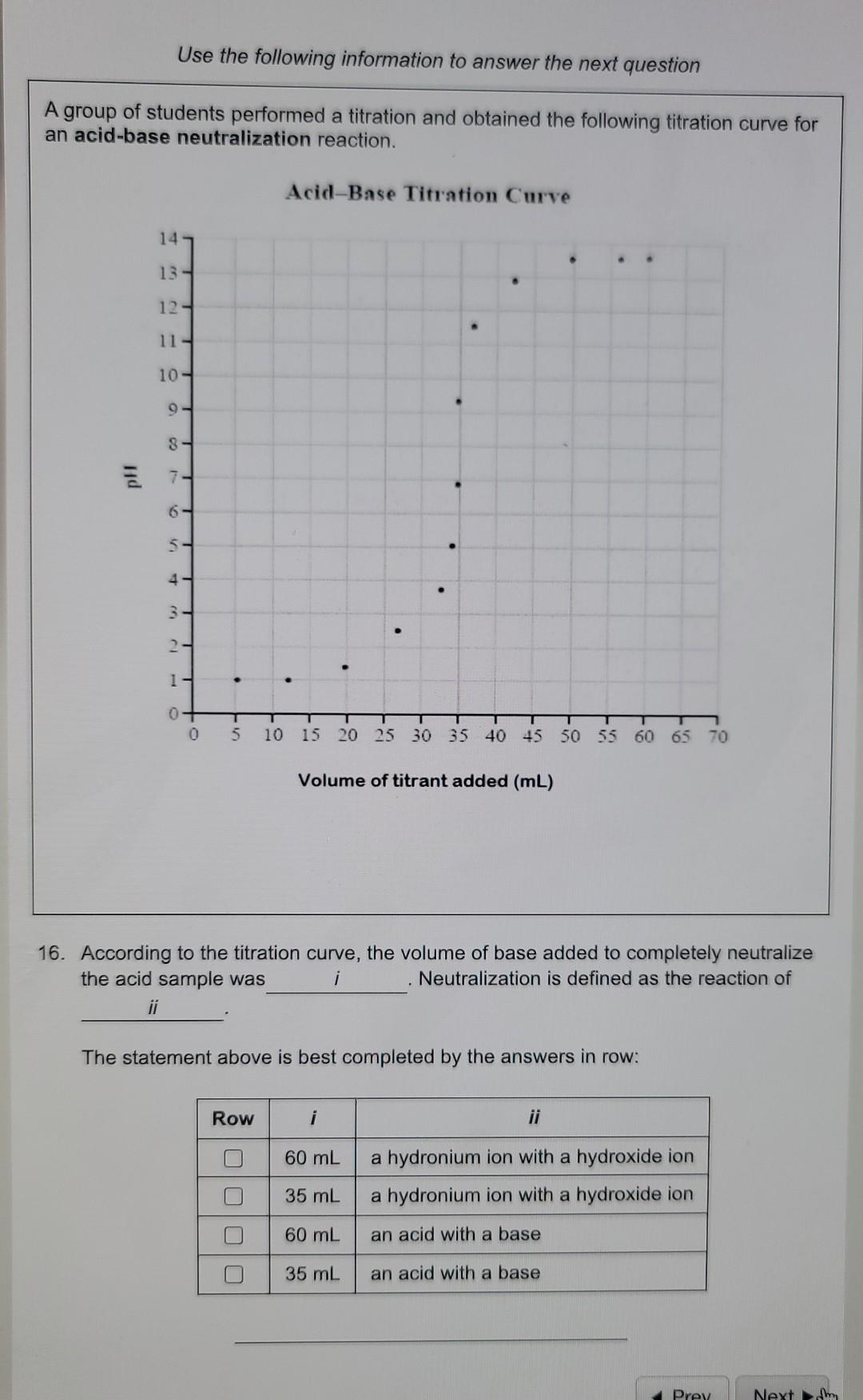
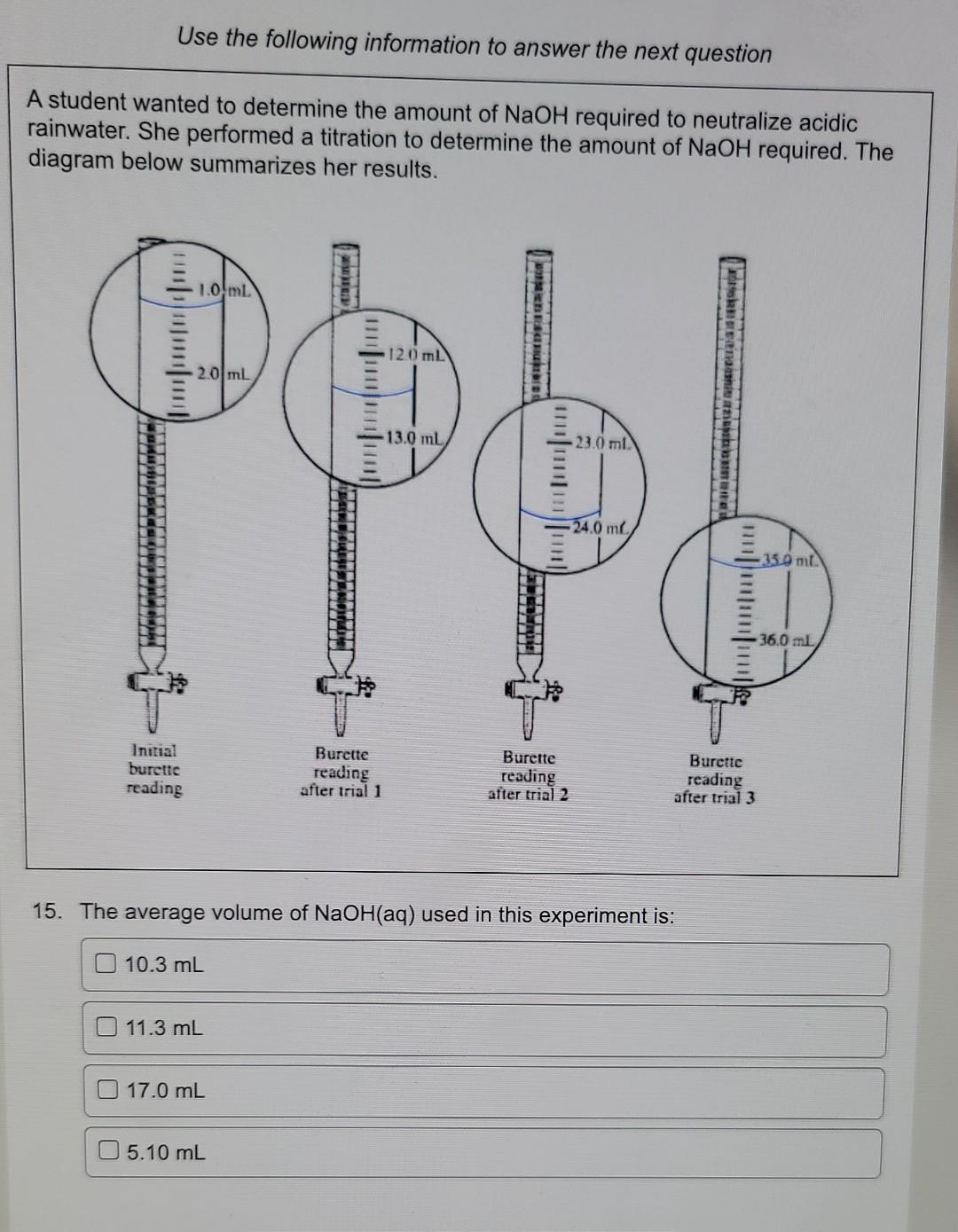
Use the following information to answer the next question A group of students performed a titration and obtained the following titration curve for an acid-base neutralization reaction. Acid-Base Titration Curve 14 13- 12 11 - 10- 9 8 - 6 4 5 - 5 - 1 - - - 3 ce 2 1 T 0 0 T 5 10 15 20 25 30 35 40 45 50 55 60 65 70 Volume of titrant added (mL) 16. According to the titration curve, the volume of base added to completely neutralize the acid sample was i Neutralization is defined as the reaction of ii The statement above is best completed by the answers in row: Row i 60 mL a hydronium ion with a hydroxide ion a hydronium ion with a hydroxide ion 35 mL O O 60 mL an acid with a base 35 mL an acid with a base Prey Next Use the following information to answer the next question A student wanted to determine the amount of NaOH required to neutralize acidic rainwater. She performed a titration to determine the amount of NaOH required. The diagram below summarizes her results. 1.0 mL 120 mL Renauha 2.0 mL 13.0 mL 23.0 ml -24.me 35.0 mt. MOD 36.0 ml Initial burcttc reading Burette reading after trial 1 Burette reading after trial 2 Burette reading after trial 3 15. The average volume of NaOH(aq) used in this experiment is: 10.3 mL 11.3 mL 17.0 mL 5.10 mL
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


