Question: Use the following information to answer the question. The pH of a solution is given by the formula pH = '0910 W] where [H+] is
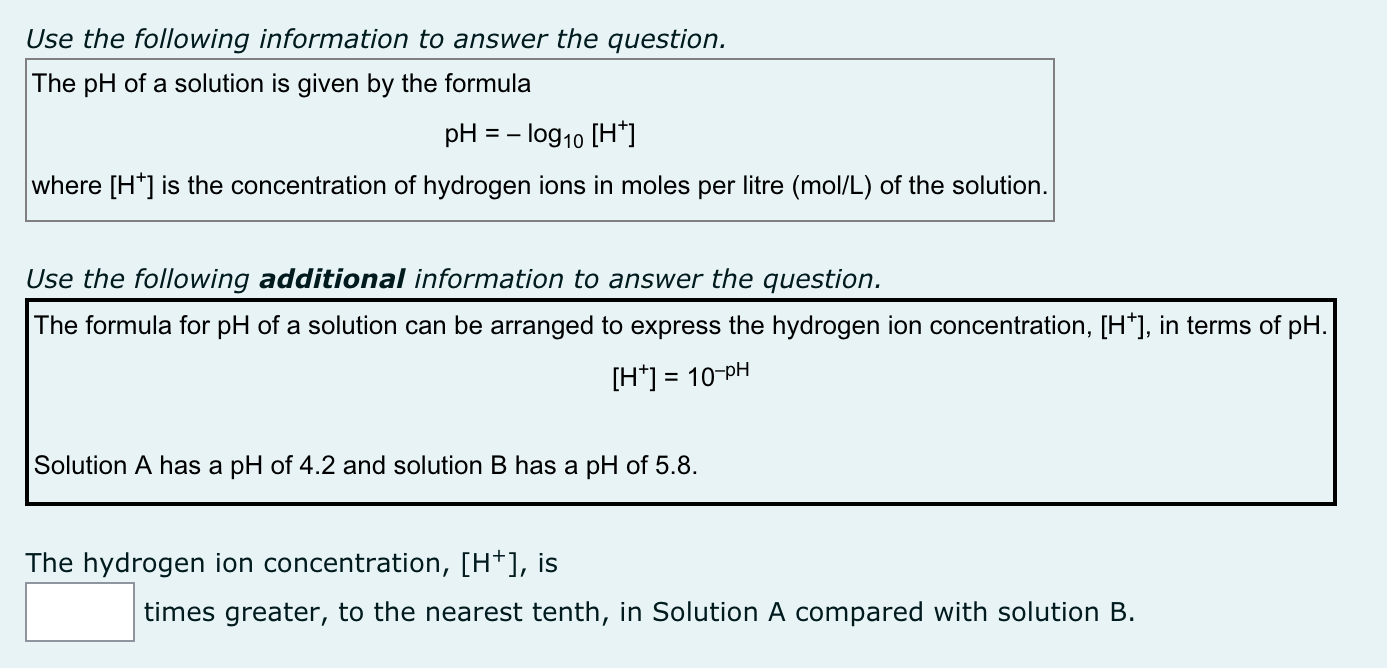
Use the following information to answer the question. The pH of a solution is given by the formula pH = '0910 W] where [H+] is the concentration of hydrogen ions in moles per litre (moliL) of the solution. Use the foiiowing additional information to answer the question. The formula for pH of a solution can be arranged to express the hydrogen ion concentration, [H+], in terms of pH. [H+] = 10-pH Solution A has a pH of 4.2 and solution B has a pH of 5.8. The hydrogen ion concentration, [H+], is |:| times greater, to the nearest tenth, in Solution A compared with solution B
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


