Question: Use the image below to answer today's google form check in. Use the table from today's notes for bond energies. Calculate the energy involved in
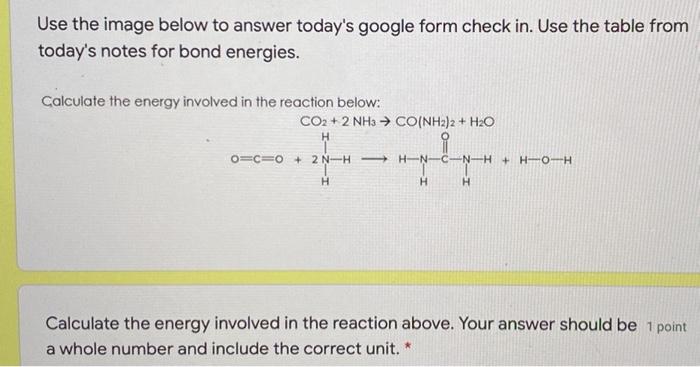
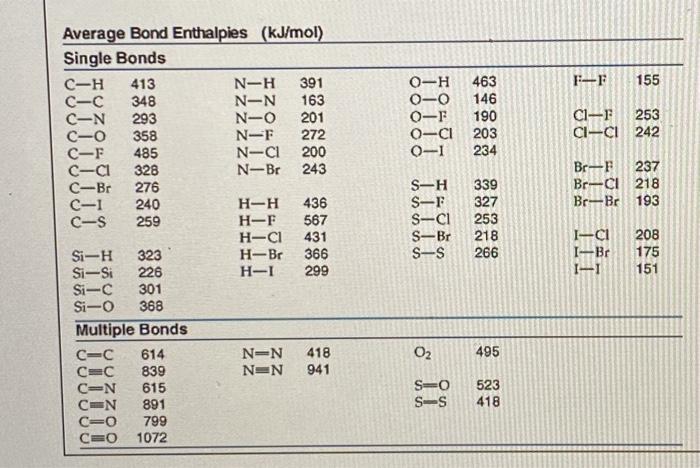
Use the image below to answer today's google form check in. Use the table from today's notes for bond energies. Calculate the energy involved in the reaction below: CO2 + 2 NH3 CO(NH2)2 + H2O H C-N-H+ H-O-H O=C=0 + 2N-H- H-N H H H Calculate the energy involved in the reaction above. Your answer should be 1 point a whole number and include the correct unit.* FI 155 O-H O-O 0-F 0-CI O-1 463 146 190 203 234 C1- 253 CI-CI 242 Br-F 237 Br--CI 218 Br-Br 193 Average Bond Enthalpies (kJ/mol) Single Bonds C-H 413 N-H 391 C-C 348 N-N 163 C-N 293 N-O 201 C-0 358 N-F 272 C-F 485 N-C 200 C-C1 328 N-Br 243 C-Br 276 C-1 240 H-H 436 c-s 259 H-F 567 H-C1 431 Si-H 323 H-Br 366 Si-Si 226 H- 299 Si-C 301 Si-O 368 Multiple Bonds C=C 614 NEN 418 C=C 839 NON 941 CON 615 CON 891 CEO 799 C=0 1072 S-H S-F S-CI S-Br S-S 339 327 253 218 266 I-CI I--BC 1-1 208 175 151 02 495 S0 S-S 523 418
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


