Question: Use the interactive to help you answer the question Select Reaction An 2 A(g) + heat = B(g) -1 0 +1 endo CRO Q=1.00 4.00
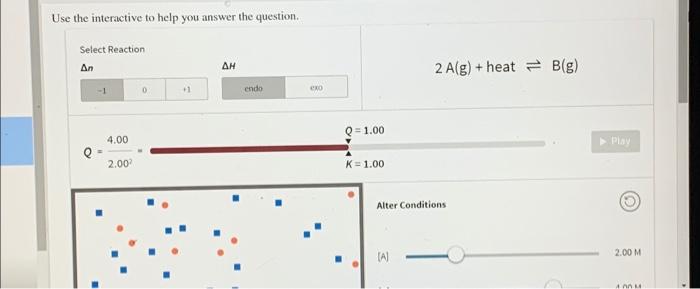
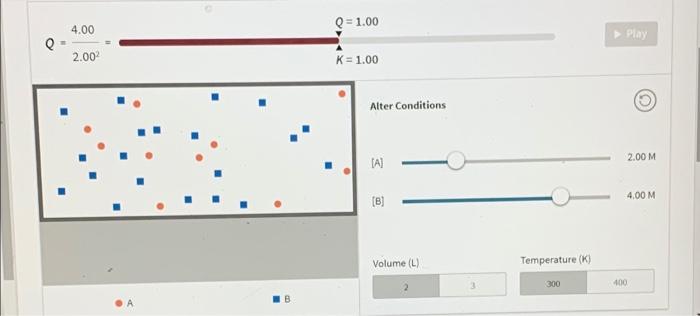
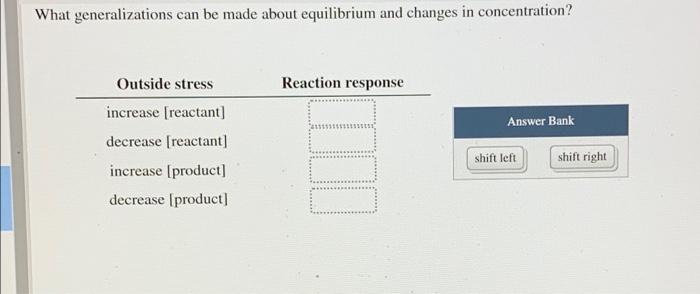

Use the interactive to help you answer the question Select Reaction An 2 A(g) + heat = B(g) -1 0 +1 endo CRO Q=1.00 4.00 Play 2.00 K=1.00 Alter Conditions (A) 2.00 M Q = 1.00 4.00 Play 2.00 K = 1.00 Alter Conditions 2.00 M (A) 4.00 M [B] Volume (L) Temperature (K) 300 400 What generalizations can be made about equilibrium and changes in concentration? Reaction response Answer Bank Outside stress increase (reactant) decrease [reactant] increase (product) decrease (product] shift left shift right After changing the concentrations of A and B, they re-adjust but eventually stop. Why? The reaction stops once it reaches equilibrium. The forward and reverse reactions occur at the same rate, keeping the concentrations constant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


