Question: Use the lab introduction to answer the following question. Answer all questions with proper significant figures. When entering units, use proper abbreviated units with proper
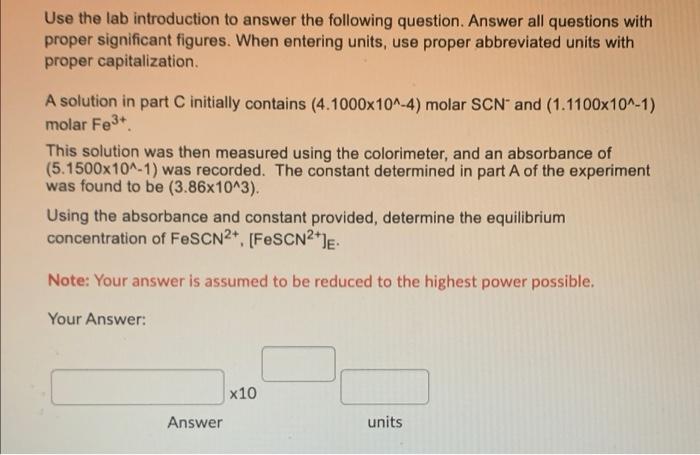
Use the lab introduction to answer the following question. Answer all questions with proper significant figures. When entering units, use proper abbreviated units with proper capitalization. A solution in part C initially contains (4.1000x10^-4) molar SCN and (1.1100x10^-1) molar Fe3+ This solution was then measured using the colorimeter, and an absorbance of (5.1500x10^-1) was recorded. The constant determined in part A of the experiment was found to be (3.86x10^3). Using the absorbance and constant provided, determine the equilibrium concentration of FeSCN2+ [FeSCN2le. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: x10 Answer units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


