Question: Use the observation in the first column to answer the question in the second column. observation question Which has the higher boiling point? The enthalpy
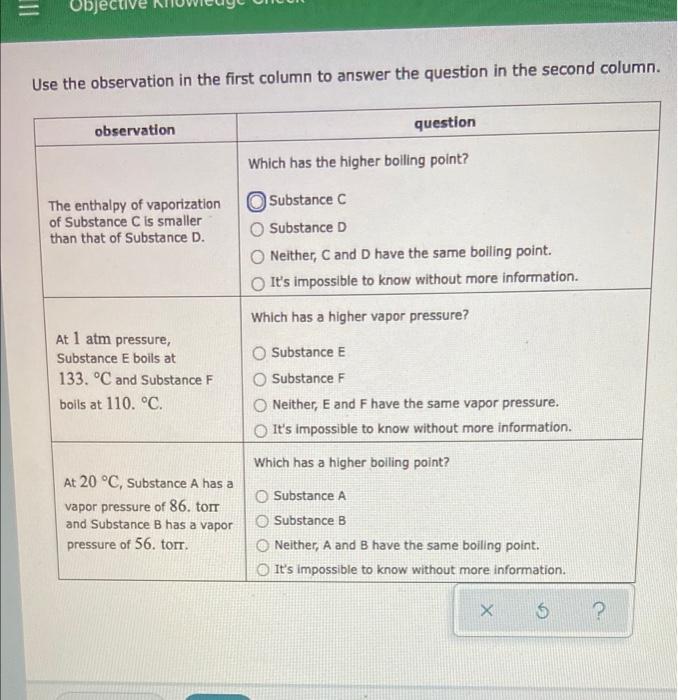
Use the observation in the first column to answer the question in the second column. observation question Which has the higher boiling point? The enthalpy of vaporization Substance C of Substance C is smaller than that of Substance D. Substance D Neither, C and D have the same boiling point. It's impossible to know without more information. Which has a higher vapor pressure? At 1 atm pressure, Substance E boils at 133. C and Substance F boils at 110. C. O Substance E Substance F Neither, E and F have the same vapor pressure. It's impossible to know without more information. Which has a higher boiling point? At 20 C. Substance A has a vapor pressure of 86. torr and Substance B has a vapor pressure of 56. torr. Substance A Substance B Neither, A and B have the same boiling point. It's impossible to know without more information. 5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


