Question: Use the phase diagram to answer the question Normal boiling point, C 30% is the lowest possible concentration of benzene to still get a vapour
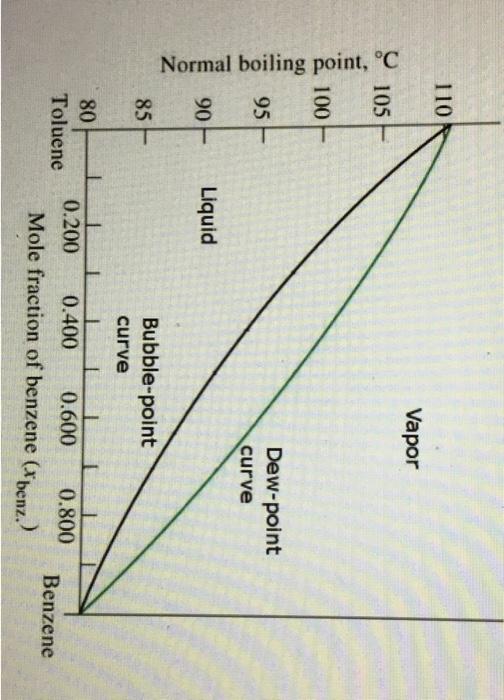
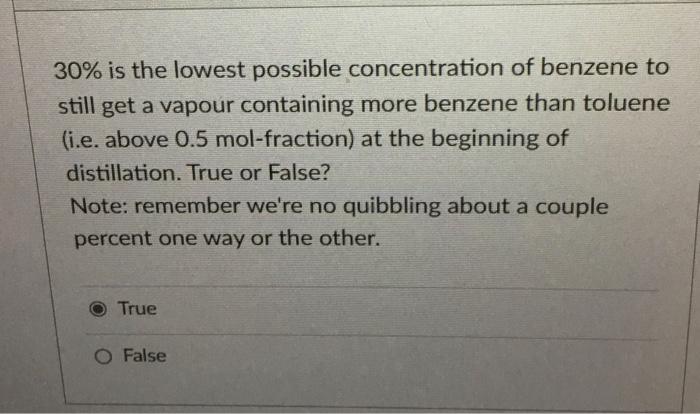
Normal boiling point, C 30% is the lowest possible concentration of benzene to still get a vapour containing more benzene than toluene (i.e. above 0.5mol-fraction) at the beginning of distillation. True or False? Note: remember we're no quibbling about a couple percent one way or the other. True False
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


