Question: Use the Reference to access importante A 13.7 g sample of an aqueous solution of hydrobromle acid contains an unknown amount of the acid If

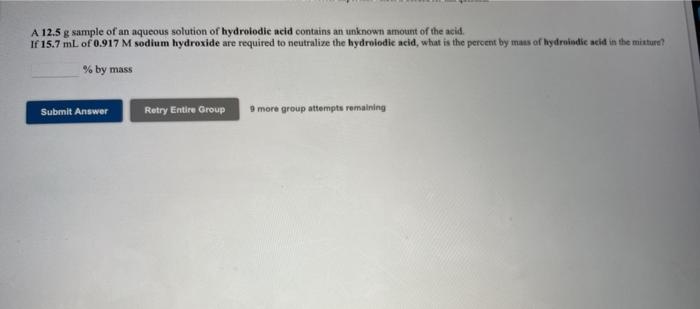
Use the Reference to access importante A 13.7 g sample of an aqueous solution of hydrobromle acid contains an unknown amount of the acid If 17.5 mL of 0.496 M potassium hydroxide are required to neutralize the hydrobromic acid, what is the percent by mass of hydrobumised in the mixture? % by mass Submit Answer Retry Entire Group 9 more group attempts remaining A 12.5 g sample of an aqueous solution of hydrolodic acid contains an unknown amount of the acid. If 15.7 mL of 0.917 M sodium hydroxide are required to neutralize the hydrolodic acid, what is the percent by mass of hydroindic acid in the mixture? % by mass Submit Answer Retry Entire Group 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


