Question: Use the References to access impertant vabes if needed for this questien. Pb2++Mg2++2H2OMg+PbO2+4H+ In the above redox reaction, use oxidaticn numbors to identify the element
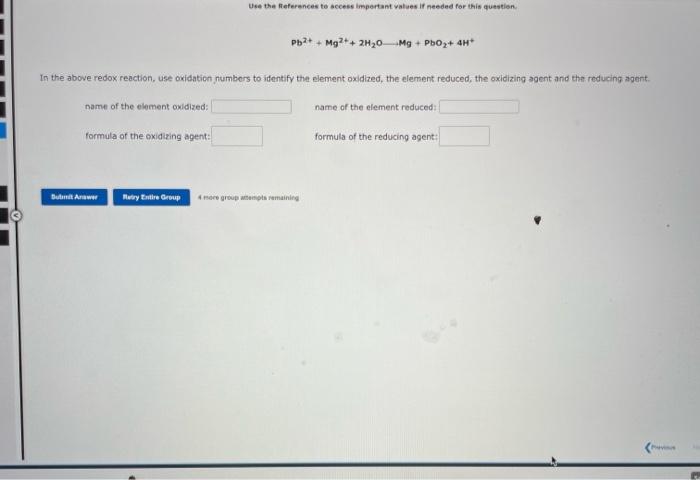
Use the References to access impertant vabes if needed for this questien. Pb2++Mg2++2H2OMg+PbO2+4H+ In the above redox reaction, use oxidaticn numbors to identify the element oxidized, the element reduced, the oxidizing agent and the reducing agant. name of the element oxidized: name of the element reduced: formula of the oxiditing agent: formula of the reducing agent
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


