Question: Use the References to access important values if needed for this question. Br+3SO42+6H+3H2SO3+BrO3 In the above redox reaction, use oxidation numbers to identify the element
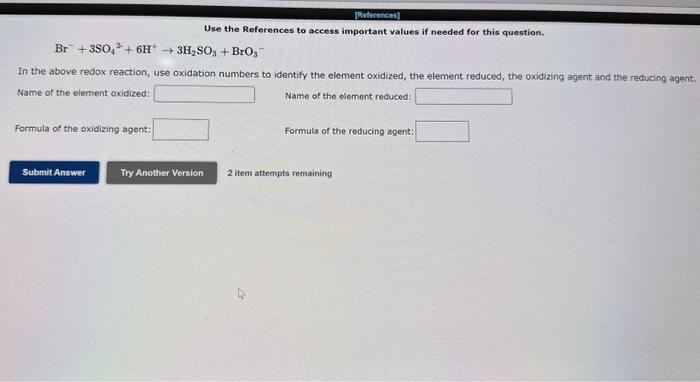
Use the References to access important values if needed for this question. Br+3SO42+6H+3H2SO3+BrO3 In the above redox reaction, use oxidation numbers to identify the element oxidized, the element reduced, the oxidizing agent and the reducing agent. Name of the element oxidized: Name of the element reduced: Formula of the oxidizing agent: Formula of the reducing agent: 2 item attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


