Question: Use the References to access important values if needed for this question. A bomb calorimeter, or a constant volume calorimeter, is a device often used
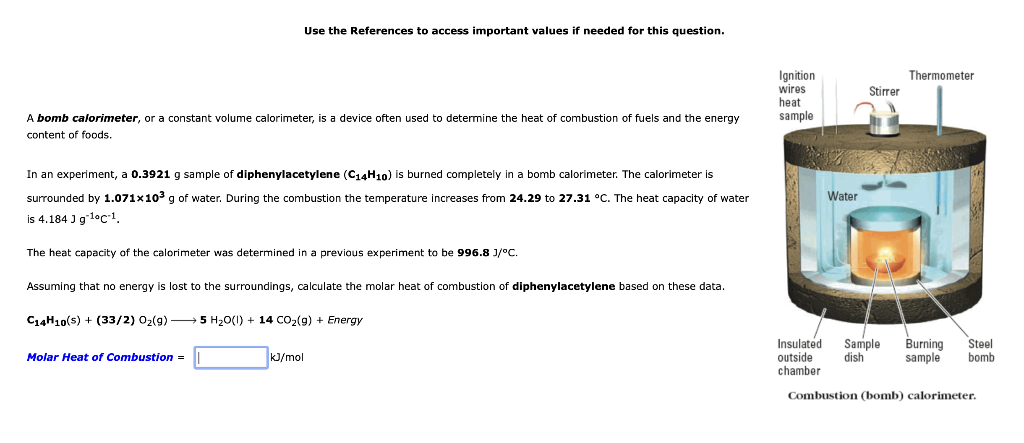
Use the References to access important values if needed for this question. A bomb calorimeter, or a constant volume calorimeter, is a device often used to determine the heat of combustion of fuels and the energy content of foods. In an experiment, a 0.3921g sample of diphenylacetylene (C14H10) is burned completely in a bomb calorimeter. The calorimeter is surrounded by 1.071103g of water. During the combustion the temperature increases from 24.29 to 27.31C. The heat capacity of water is 4.184Jg1CC1. The heat capacity of the calorimeter was determined in a previous experiment to be 996.8J/C. Assuming that no energy is lost to the surroundings, calculate the molar heat of combustion of diphenylacetylene based on these data. C14H10(s)+(33/2)O2(g)5H2O(I)+14CO2(g)+Energy Molar Heat of Combustion = kJ/mol Combustion (bomb) calorimeter
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


