Question: Uve the References to access important values if needed for this question. A bomb calorimeter, or constant volume calorimeter, is a device c ften used
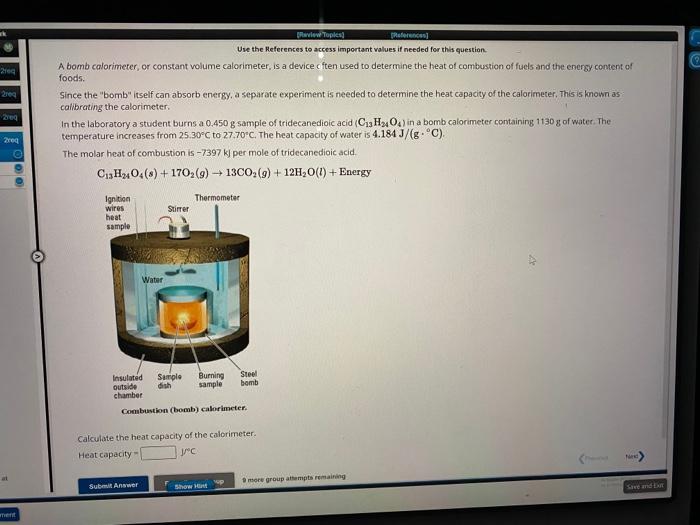
Uve the References to access important values if needed for this question. A bomb calorimeter, or constant volume calorimeter, is a device c ften used to determine the heat ot combuskion of fuels and the enersy content of foods. Since the "bomb" itself can absorb energy, a separate experiment is needed to determine the heat capacity of the calorimeter. This is known as callbrating the calorimcter. In the laboratory a student burns a 0,450g sample of tridecanedioic acid (C13H24O4 ) in a bomb calorimeter containing 1130 g of water. The temperature increases from 25.30C to 27.70C. The heat capacity of water is 4.184J/(gC). The molar heat of combustion is 7397kj per mole of tridecanedioic acid. C13H24O4(s)+17O2(g)13CO2(g)+12H2O(l)+Energy Conbustion (bomb) calorimeter. Calculate the heat capacity of the calorimeter. Heat capacity = Uve the References to access important values if needed for this question. A bomb calorimeter, or constant volume calorimeter, is a device c ften used to determine the heat ot combuskion of fuels and the enersy content of foods. Since the "bomb" itself can absorb energy, a separate experiment is needed to determine the heat capacity of the calorimeter. This is known as callbrating the calorimcter. In the laboratory a student burns a 0,450g sample of tridecanedioic acid (C13H24O4 ) in a bomb calorimeter containing 1130 g of water. The temperature increases from 25.30C to 27.70C. The heat capacity of water is 4.184J/(gC). The molar heat of combustion is 7397kj per mole of tridecanedioic acid. C13H24O4(s)+17O2(g)13CO2(g)+12H2O(l)+Energy Conbustion (bomb) calorimeter. Calculate the heat capacity of the calorimeter. Heat capacity =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


