Question: Use the References to access important values if needed for this question. Hint: No calculations are required. For the reaction H2(g)+Cl2(g)2HCl(g) H=185kJandS=20.0J/K At standard conditions,
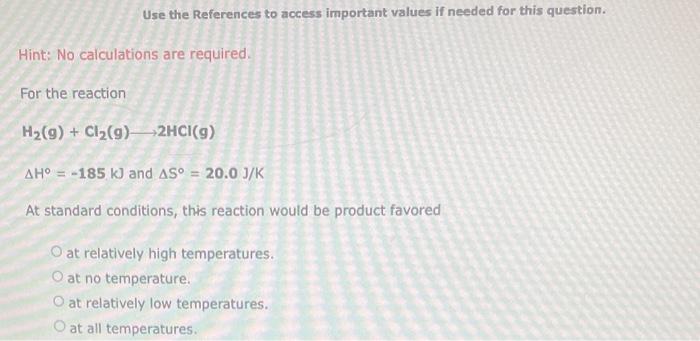
Use the References to access important values if needed for this question. Hint: No calculations are required. For the reaction H2(g)+Cl2(g)2HCl(g) H=185kJandS=20.0J/K At standard conditions, this reaction would be product favored at relatively high temperatures. at no temperature. at relatively low temperatures. at all temperatures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


