Question: Use the References to access important values if needed for this question. What volume of oxygen gas is required to react completely with 0.824 mol
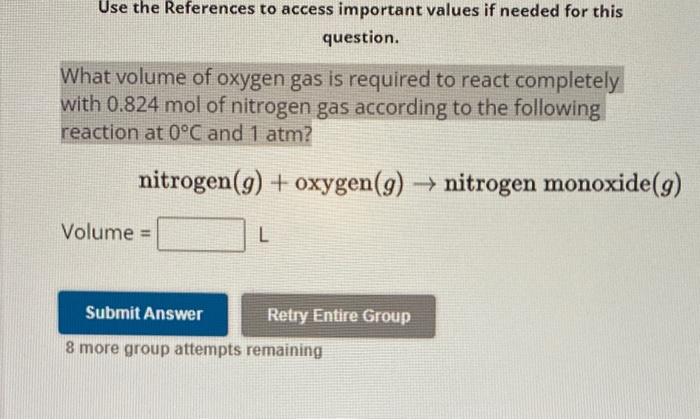

Use the References to access important values if needed for this question. What volume of oxygen gas is required to react completely with 0.824 mol of nitrogen gas according to the following reaction at 0C and 1atm ? nitrogen(g)+oxygen(g)nitrogenmonoxide(g) Volume = L 8 more group attempts remaining Use the References to access important values if needed for this question. What volume of hydrogen gas is produced when 3.13mol of carbon monoxide reacts completely according to the following reaction at 0C and 1 atm? carbon monoxide (g)+ water (I) carbon dioxide (g)+ hydrogen (g) liters hydrogen gas 14.2 8 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


