Question: Use the References to access important values if needed for this nuestion. The rearrangement of cyclopropane to propene at 500C (CH2)3CH3CH=CH2 is first order in
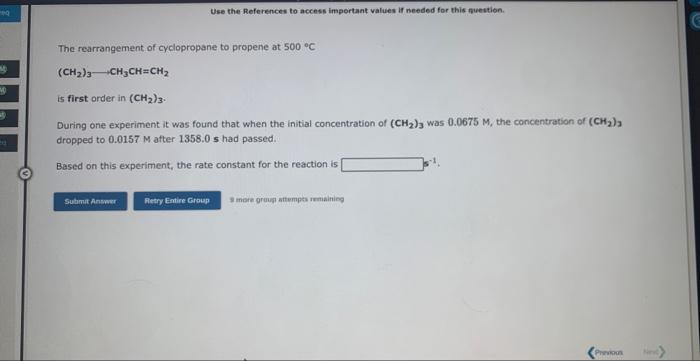
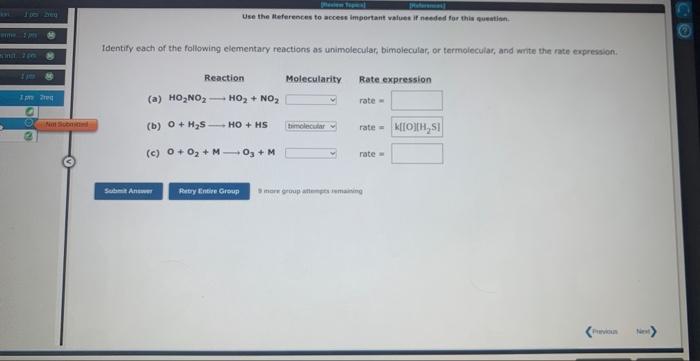
Use the References to access important values if needed for this nuestion. The rearrangement of cyclopropane to propene at 500C (CH2)3CH3CH=CH2 is first order in (CH2)3. During one experiment it was found that when the initial concentration of (CH2)3 was 0.0675M3 the concentration of (CH2)3 dropped to 0.0157M after 1358.0 s had passed. Based on this experiment, the rate constant for the reaction is 8 ince grusi attempes sumaining Use the Ateferences to accese important vafues if needed for this queatinn. fdentify each of the following elementary reactions as unimolecular, bimolecular, or termolecular, and write the rate expression
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


