Question: Use the References to access important values if needed for this question. A reaction is performed to study the reaction of nitrogen dioxide with fluorine:
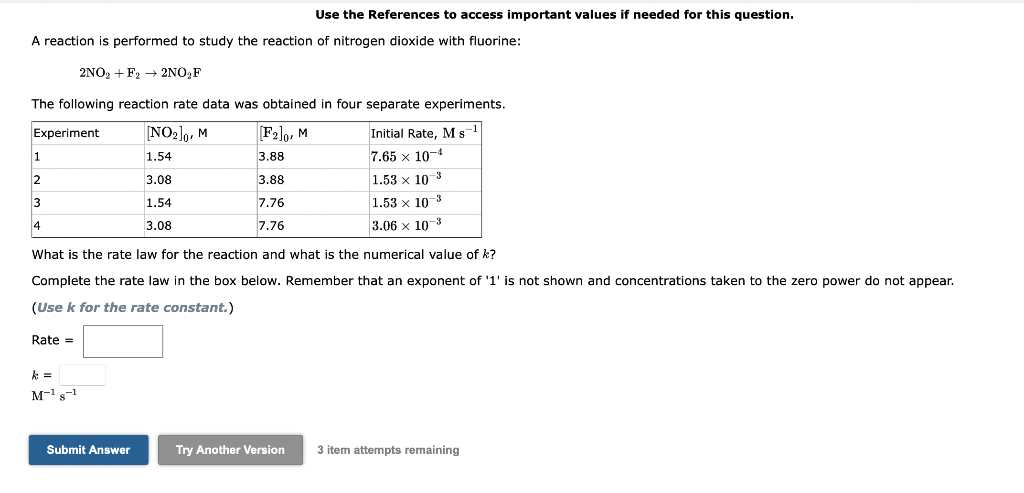

Use the References to access important values if needed for this question. A reaction is performed to study the reaction of nitrogen dioxide with fluorine: 2NO2+F22NO2F The following reaction rate data was obtained in four separate experiments. What is the rate law for the reaction and what is the numerical valu k ? Complete the rate law in the box below. Remember that an exponent of ' 1 ' is not shown and concentrations taken to the zero power do not appear. (Use k for the rate constant.) Rate = k=M1s1 3 item attempts remaining Use the References to access important values if needed for this question. The gas phase decomposition of dinitrogen pentoxide at 335K is first order in N2O5. N2O5(g)2NO2(g)+1/2O2(g) During one experiment it was found that the N2O5 concentration dropped from 6.75102M at the beginning of the experiment to 2.07102M in 279 s. What is the value of the rate constant for the reaction at this temperature? s1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


