Question: Use the References to access important values if needed for this question. Write an equation that shows the formation of the phosphide ion from a
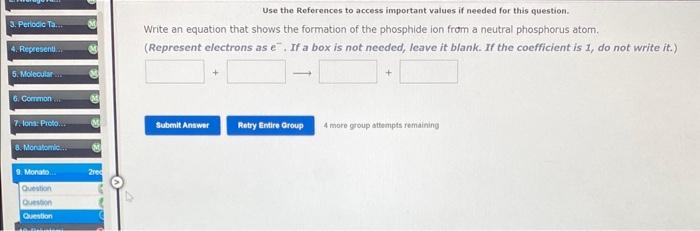
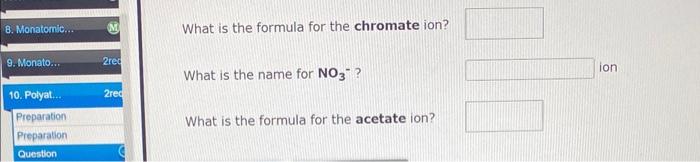
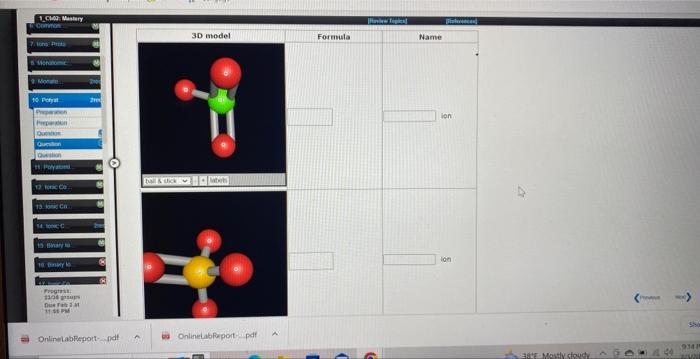
Use the References to access important values if needed for this question. Write an equation that shows the formation of the phosphide ion from a neutral phosphorus atom. (Represent electrons as e. If a box is not needed, leave it blank. If the coefficient is 1 , do not write it.) 4 more group attompts remaining: What is the formula for the chromate ion? What is the name for NO3? ion What is the formula for the acetate ion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


