Question: Use the References to access important values if needed for this nunstion. Considering only ions with charges of +1,+2,1 and 2, or neutral atoms, give
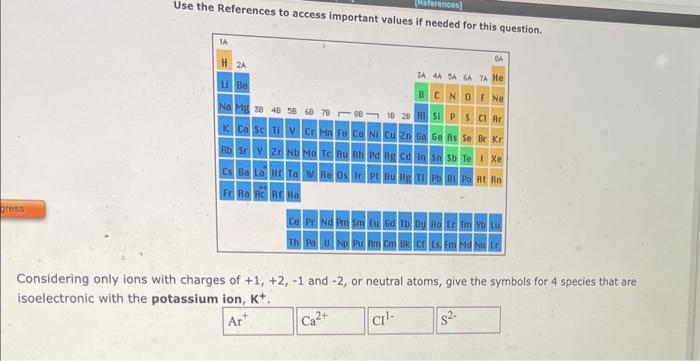
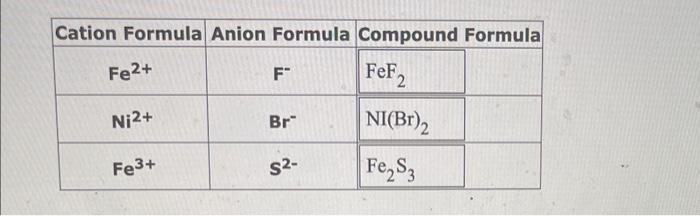

Use the References to access important values if needed for this nunstion. Considering only ions with charges of +1,+2,1 and 2, or neutral atoms, give the symbols for 4 species that are isoelectronic with the potassium ion, K+. \begin{tabular}{|c|c|l|} \hline Cation Formula & Anion Formula & \multicolumn{2}{|c|}{ Compound Formula } \\ \hline Fe2+ & F & FeF2 \\ \hline Ni2+ & Br & NI(Br2 \\ \hline Fe3+ & S2 & Fe2S3 \\ \hline \end{tabular} formula for the compound formed between each of the pairs of ions given in the table
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


