Question: Use the References to access important values if needed for this question. According to the following reaction, how many moles of hydrochloric acid are necessary

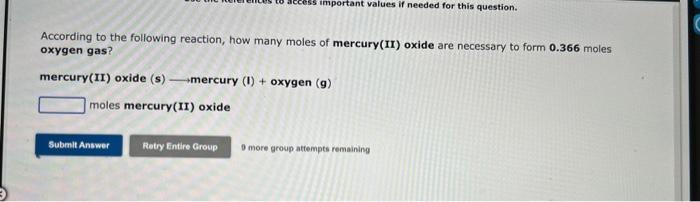
Use the References to access important values if needed for this question. According to the following reaction, how many moles of hydrochloric acid are necessary to form 0.980 moles calcium chloride? calcium hydroxide (aq) + hydrochloric acid (aq) __calcium chloride (aq) + water (I) moles hydrochloric acid According to the following reaction, how many moles of mercury(II) oxide are necessary to form 0.366moles oxygen gas? mercury (II) oxide (s) mercury (I)+ oxygen (g) moles mercury(II) oxide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


