Question: Use the References to access important values if needed for this question. The vapor pressure of diethyl ether is 400mmHg at 18.0C. From the plot
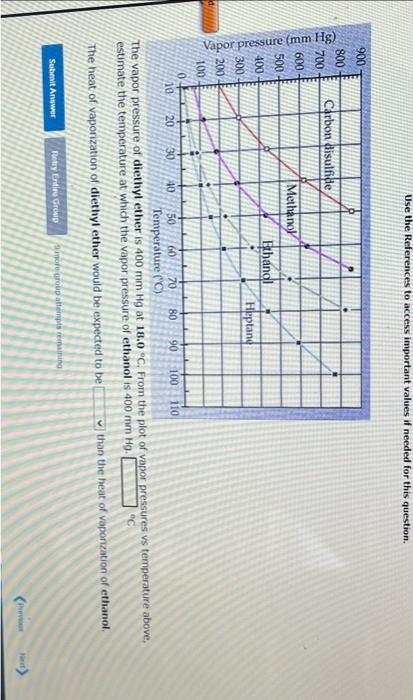
Use the References to access important values if needed for this question. The vapor pressure of diethyl ether is 400mmHg at 18.0C. From the plot of vador pressures vs temperature above. estimate the temperature at which the vapor pressure of ethanol is 400mmHg. The heat of vaporization of diethyl ether would be expected to be than the heat of vaporization of ethanol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


