Question: Use the References to access important values if needed for this question. A student weighs out a 2.50 g sample of CuF2, transfers it to
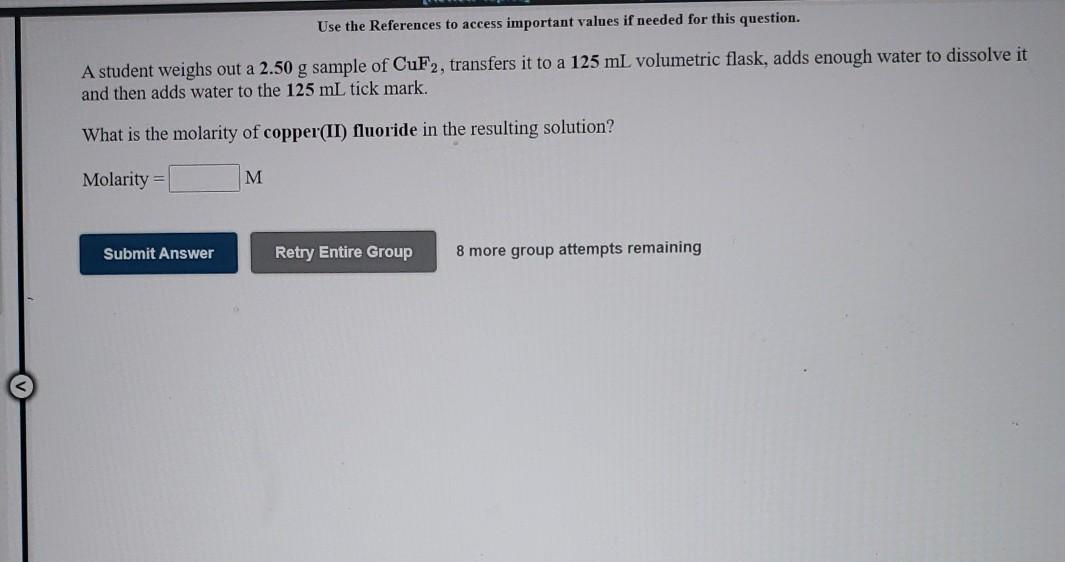

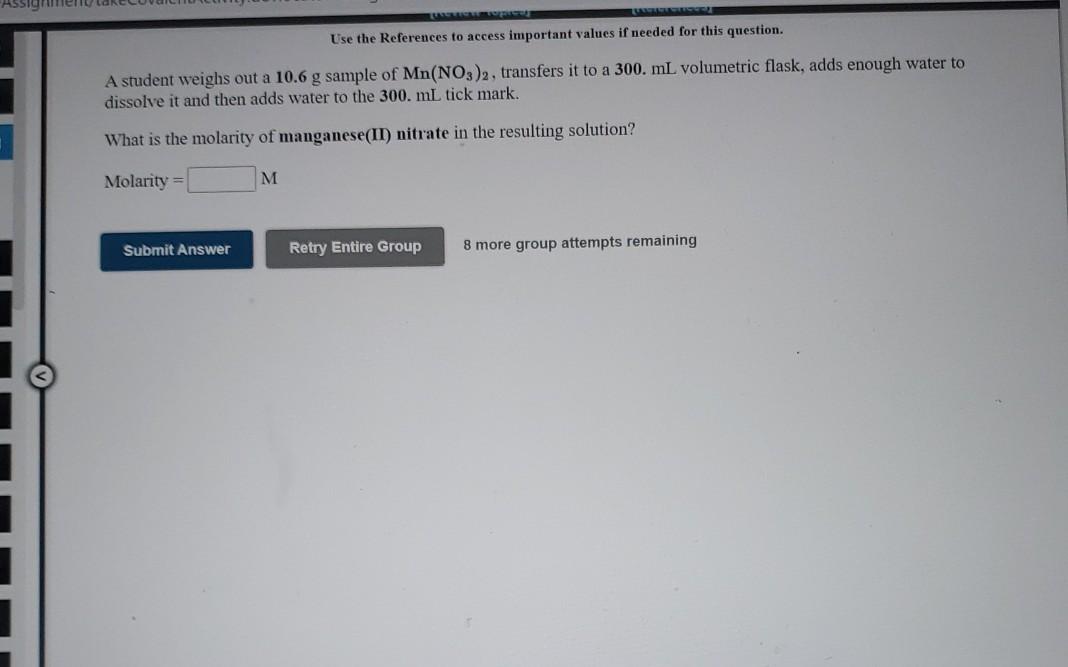
Use the References to access important values if needed for this question. A student weighs out a 2.50 g sample of CuF2, transfers it to a 125 mL volumetric flask, adds enough water to dissolve it and then adds water to the 125 mL tick mark. What is the molarity of copper(II) fluoride in the resulting solution? Molarity = M Submit Answer Retry Entire Group 8 more group attempts remaining Use the References to access important values if needed for this question. A student weighs out a 7.13 g sample of FeBrz, transfers it to a 125 mL volumetric flask, adds enough water to dissolve it and then adds water to the 125 mL tick mark. What is the molarity of iron(III) bromide in the resulting solution? Molarity = M Submit Answer Retry Entire Group 8 more group attempts remaining Assigen Use the References to access important values if needed for this question. A student weighs out a 10.6 g sample of Mn(NO3)2, transfers it to a 300. mL volumetric flask, adds enough water to dissolve it and then adds water to the 300. mL tick mark. What is the molarity of manganese(II) nitrate in the resulting solution? Molarity = M M Submit Answer Retry Entire Group 8 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


