Question: Use the References to access important values if needed for this question. The activation energy for the gas phase isomerization of cis-2-butene is 75.3kJ. cisCH3CH=CHCH3trans-CH3CH=CHCH3
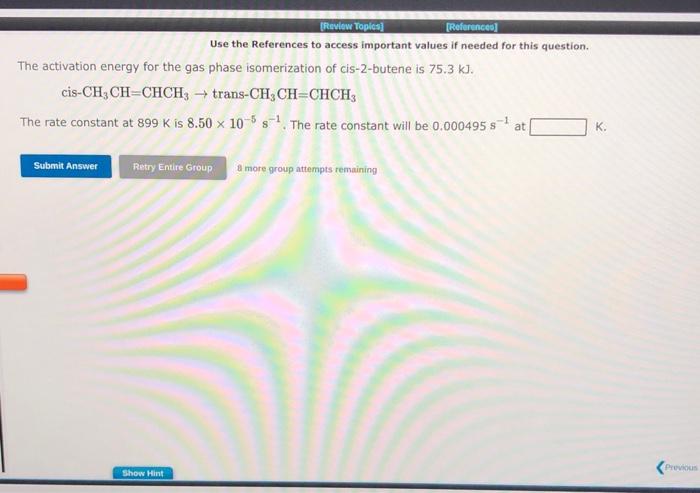
Use the References to access important values if needed for this question. The activation energy for the gas phase isomerization of cis-2-butene is 75.3kJ. cisCH3CH=CHCH3trans-CH3CH=CHCH3 The rate constant at 899K is 8.50105s1. The rate constant will be 0.000495s1 at K. 8 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


