Question: Use the References to access impertant values if needed for this question. For the gas phase isomerization of cis-cyanostyrene, cisC6H5CH=CHCNtranC6H5CH=CHCN the rate constant has been
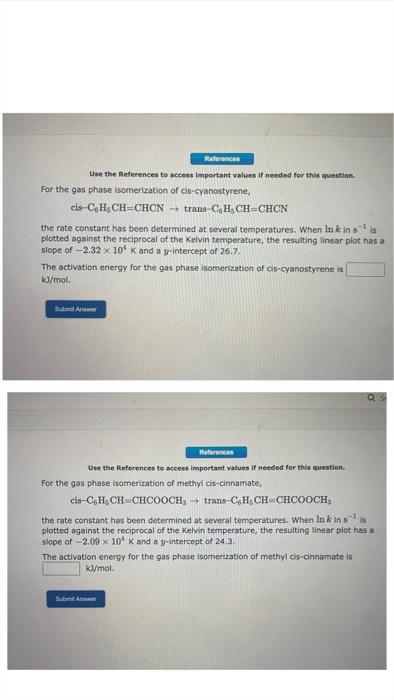
Use the References to access impertant values if needed for this question. For the gas phase isomerization of cis-cyanostyrene, cisC6H5CH=CHCNtranC6H5CH=CHCN the rate constant has been determined at several temperatures. When lnk in 51. is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of 2.32104K and a y-intercept of 26.7. The activation energy for the gas phase isomerization of cis-cyanostyrene is kj/mol. Use the References to access important values if needed for this question. For the gas phase isomerization of methyl cis-cinnamate, cisC6H5CH=CHCOOCH3transC6H5CH=CHCOOCH3 the rate constant has been determined at several temperatures. When lnkln1 is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of 2.09104K and a y-intercept of 24.3. The activation energy for the gas phase isomerization of methyl cis-cinnamate is k/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


