Question: Use the References to access important values if needed for this question. For the reaction below: CH CH2 a. HCI a. Estimate the gas phase
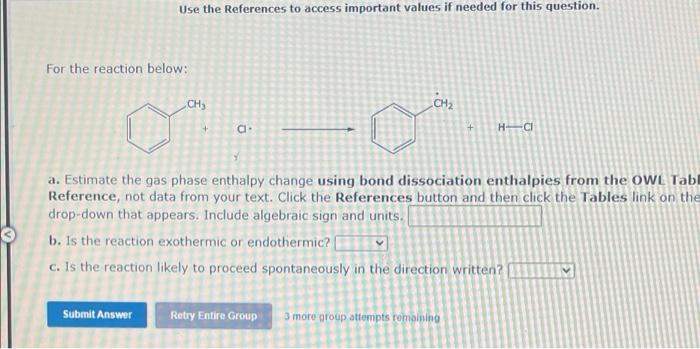
Use the References to access important values if needed for this question. For the reaction below: CH CH2 a. HCI a. Estimate the gas phase enthalpy change using bond dissociation enthalpies from the OWL Tab Reference, not data from your text. Click the References button and then click the Tables link on the drop-down that appears. Include algebraic sign and units. b. Is the reaction exothermic or endothermic? c. Is the reaction likely to proceed spontaneously in the direction written? Submit Answer Retry Entire Group 3 more group attempts romaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


