Question: Use the References to access important values if needed for this question. Radioactive decay can be described by the following equation lnA=lnA0kt where A0 is
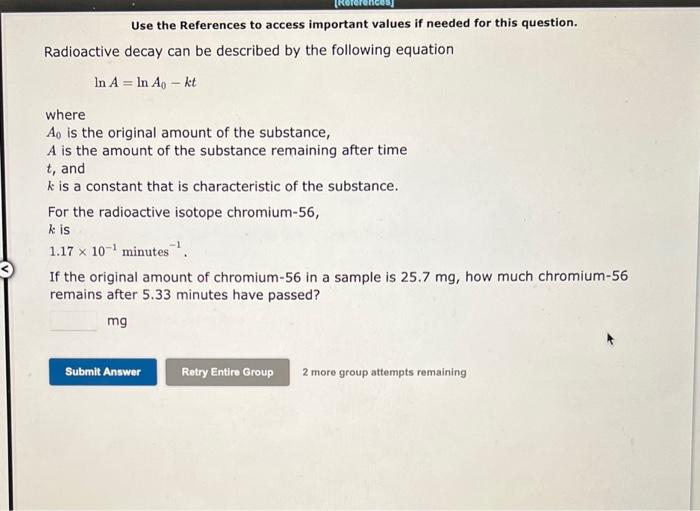
Use the References to access important values if needed for this question. Radioactive decay can be described by the following equation lnA=lnA0kt where A0 is the original amount of the substance, A is the amount of the substance remaining after time t, and k is a constant that is characteristic of the substance. For the radioactive isotope chromium-56, k is 1.17101 minutes 1 If the original amount of chromium-56 in a sample is 25.7mg, how much chromium-56 remains after 5.33 minutes have passed? mg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


