Question: Use the References to access important values il needed for The pH scale was designed to make it convenient to express hydrogen ion concentrations that
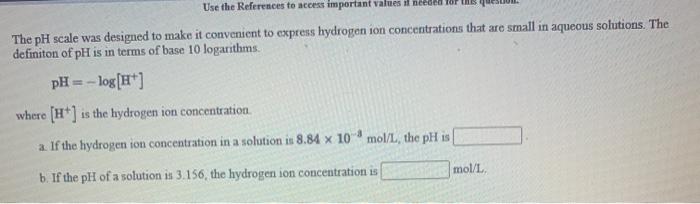

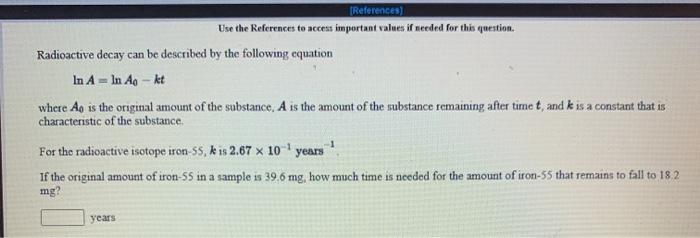
Use the References to access important values il needed for The pH scale was designed to make it convenient to express hydrogen ion concentrations that are small in aqueous solutions. The definiton of pH is in terms of base 10 logarithms. pH = -log[H+] where (His the hydrogen ion concentration. a If the hydrogen ion concentration in a solution is 8.84 x 10 mol/L, the pH is mol/L b. If the pH of a solution is 3.156, the hydrogen ion concentration is Referenceal Use the References to access important values if needed for this question Radioactive decay can be described by the following equation In A = In Ag - kt where Ag is the onginal amount of the substance. A is the amount of the substance remaining after time t, and k is a constant that is characteristic of the substance. For the radioactive isotope strontium 90, k is 2.47 x 10 years If the original amount of strontium-90 in a sample is 53.8 mg. how much strontium-90 remains after 30.1 years have passed? mg [References) Use the References to access important values if needed for this question. Radioactive decay can be described by the following equation In A = In An - kt where Ao is the original amount of the substance. A is the amount of the substance remaining after time t, and k is a constant that is characteristic of the substance. For the radioactive isotope iron 55, k is 2.67 10 years If the original amount of iron 55 in a sample is 39.6 mg, how much time is needed for the amount of iron-55 that remains to fall to 182 mg? years
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


