Question: Use the References to access important values if needed for this question. Identify whether each species functions as a Bronsted-Lowry acid or a Brnsted-Lowry base
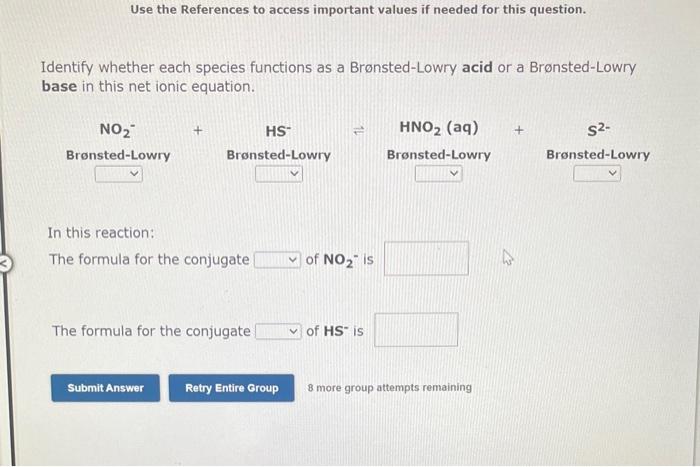
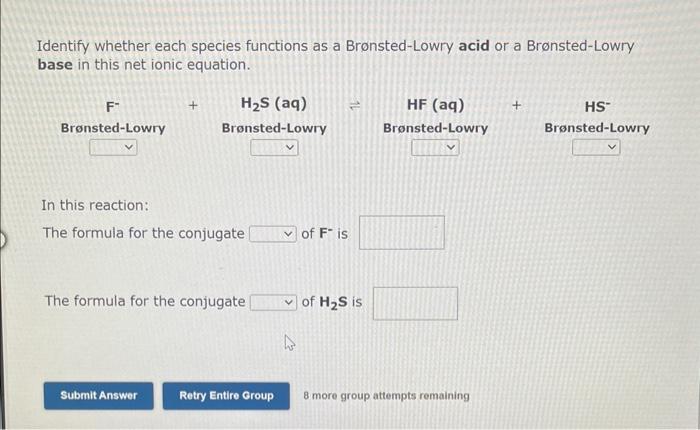
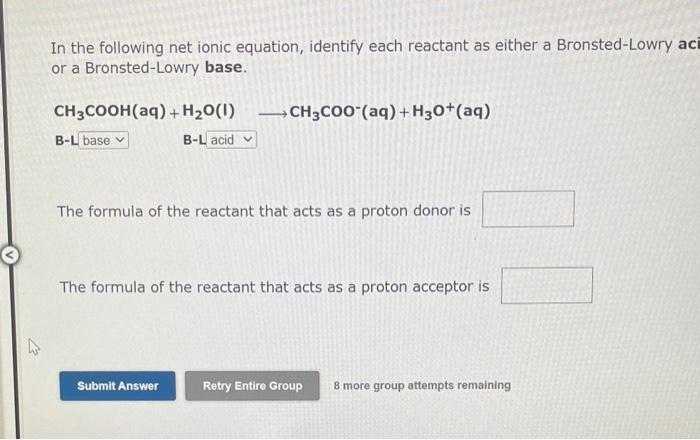
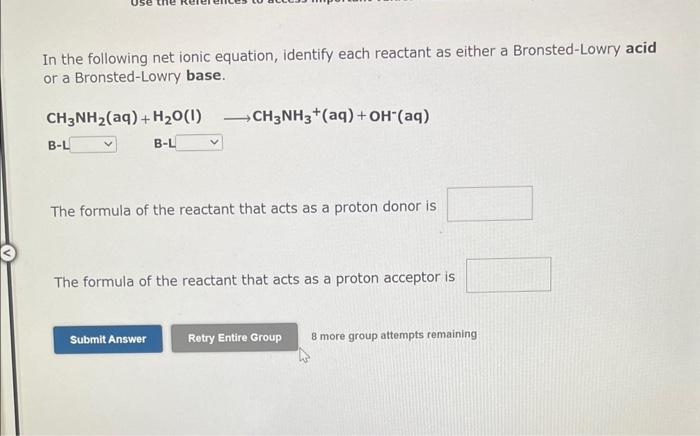
Use the References to access important values if needed for this question. Identify whether each species functions as a Bronsted-Lowry acid or a Brnsted-Lowry base in this net ionic equation. In this reaction: The formula for the conjugate of NO2is The formula for the conjugate of HSis Identify whether each species functions as a Bronsted-Lowry acid or a Bronsted-Lowry base in this net ionic equation. In this reaction: The formula for the conjugate of Fis The formula for the conjugate of H2S is In the following net ionic equation, identify each reactant as either a Bronsted-Lowry aci or a Bronsted-Lowry base. CH3COOH(aq)+H2O(I)CH3COO(aq)+H3O+(aq) B-L B-L The formula of the reactant that acts as a proton donor is The formula of the reactant that acts as a proton acceptor is 8 more group attempts remaining In the following net ionic equation, identify each reactant as either a Bronsted-Lowry acid or a Bronsted-Lowry base. CH3NH2(aq)+H2O(l)CH3NH3+(aq)+OH(aq) B-L B-L The formula of the reactant that acts as a proton donor is The formula of the reactant that acts as a proton acceptor is 8 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


