Question: the answer i submitted for the fourth picture was wrong Use the References to access important values if needed for this question. 1. The formula


 the answer i submitted for the fourth picture was wrong
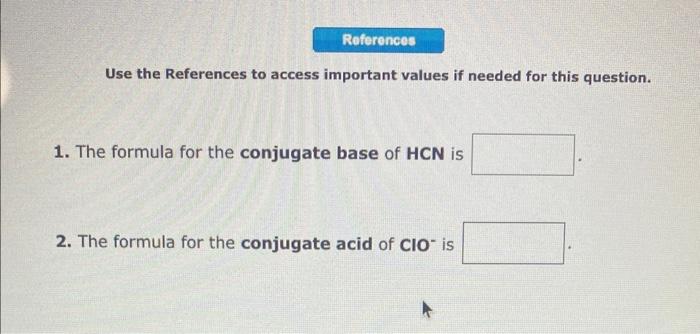
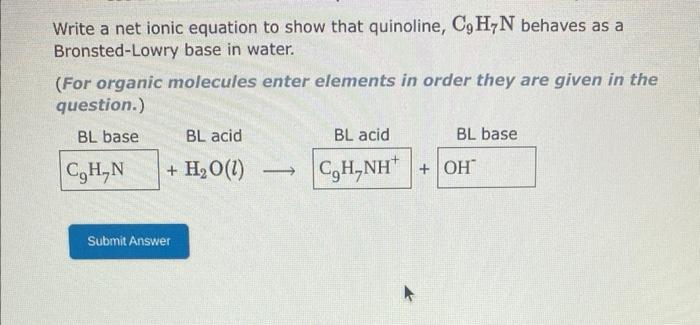
the answer i submitted for the fourth picture was wrong Use the References to access important values if needed for this question. 1. The formula for the conjugate base of HCN is 2. The formula for the conjugate acid of ClOis 1. The formula for the conjugate base of H2C6H6O6 is 2. The formula for the conjugate acid of HSO3is 1. The formula for the conjugate acid of Bris 2. The formula for the conjugate acid of NH3 is Write a net ionic equation to show that quinoline, C9H7N behaves as a Bronsted-Lowry base in water. (For organic molecules enter elements in order they are given in the question.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


