Question: Use the References to access important values if needed for this question. In the laboratory you are asked to make a 0.175m nickel(II) iodide solution
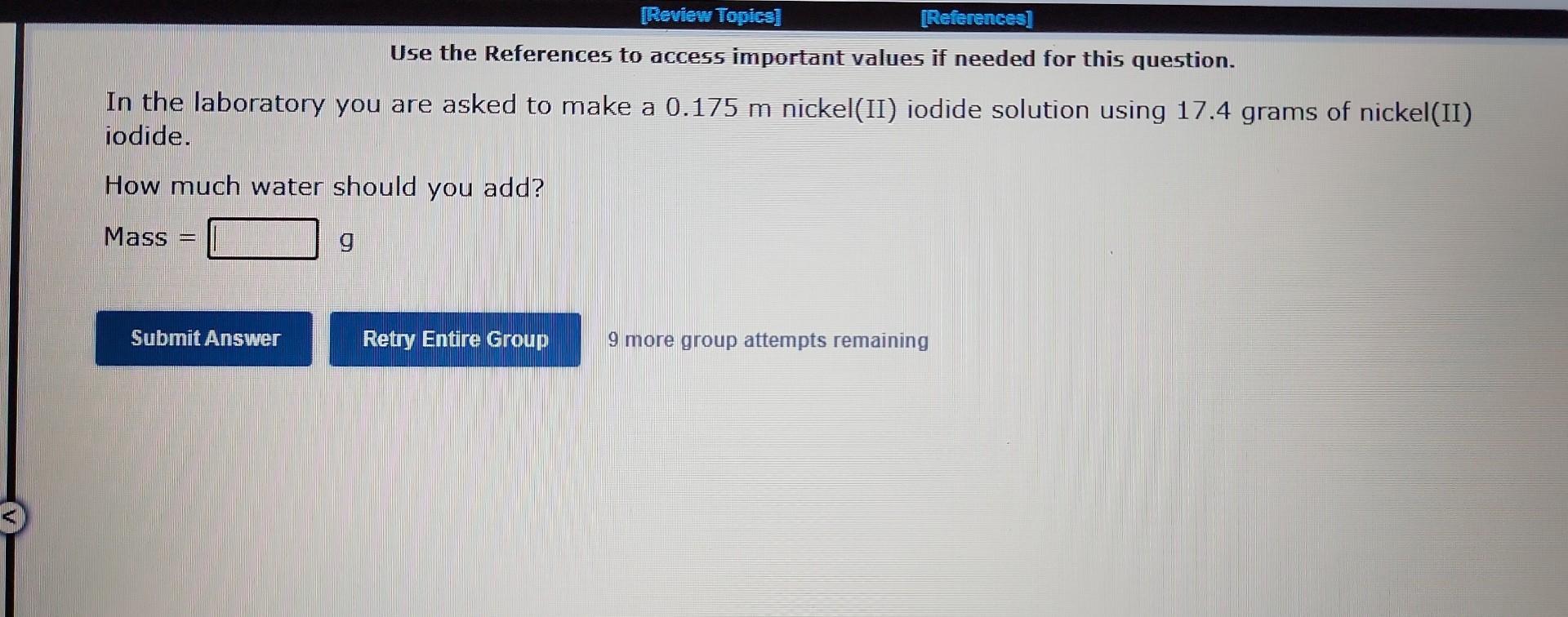
Use the References to access important values if needed for this question. In the laboratory you are asked to make a 0.175m nickel(II) iodide solution using 17.4grams of nickel(II) iodide. How much water should you add? Mass = 9
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


