Question: Use the References to access important values if needed for this question. For the following reaction, 4.28 grams of hydrogen gas are allowed to react

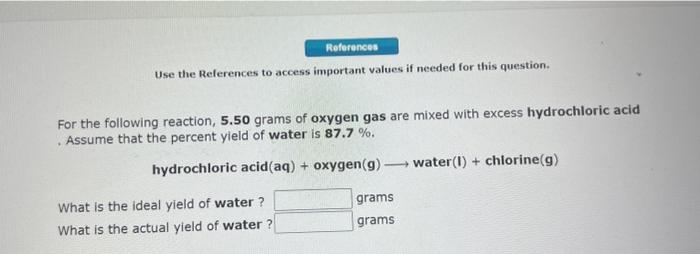
Use the References to access important values if needed for this question. For the following reaction, 4.28 grams of hydrogen gas are allowed to react with 10.4 grams of ethylene (C2H4). hydrogen (g)+ ethylene (C2H4)(g) ethane (C2H6)(g) What is the maximum mass of ethane (C2H6) that can be formed? Mass = g What is the FORMULA for the limiting reactant? What mass of the excess reagent remains after the reaction is complete? Mass = g Use the Relerences to access important values if needed for this question. For the following reaction, 5.50 grams of oxygen gas are mixed with excess hydrochloric acid . Assume that the percent yield of water is 87.7%. hydrochloric acid(aq) + oxygen (g)water(I)+ chlorine (g) What is the ideal yield of water? grams What is the actual yield of water? grams
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


