Question: Use the References to access important values if needed for this question. Caiculate the number of atoms of carbon (C) in 397cm3 of the colorless
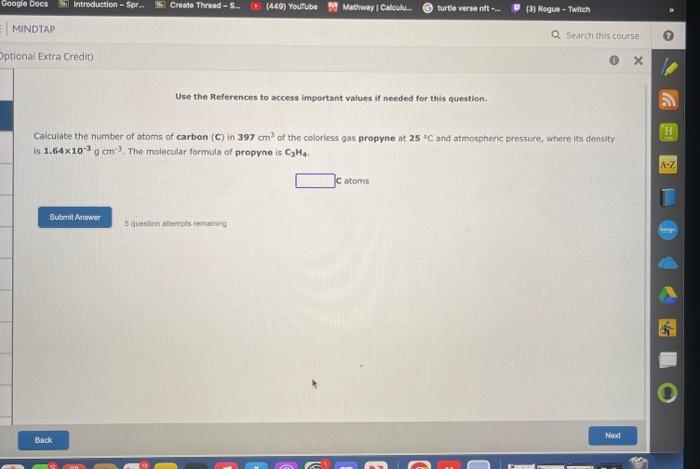
Use the References to access important values if needed for this question. Caiculate the number of atoms of carbon (C) in 397cm3 of the colorless gas propyne at 25 o C and atmospheric pressure, where its denaty is 1,64103gcm3. The molecular formule of propyne is C2H4. Catems S qussuce aflenpts ramsaining
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


