Question: Use the References to access important values if needed for this question Potassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory
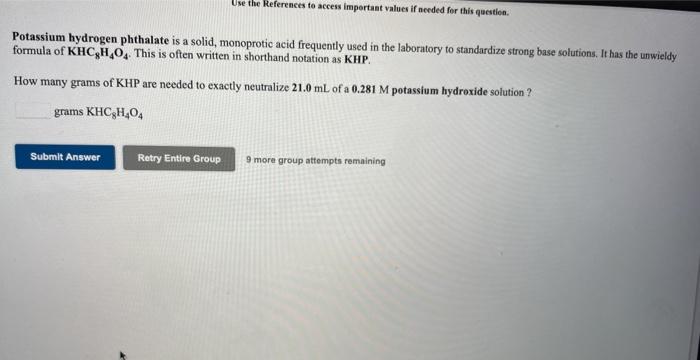
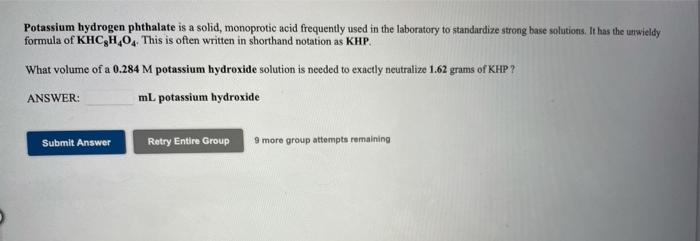
Use the References to access important values if needed for this question Potassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory to standardize strong buse solutions. It has the unwieldy formula of KHCH, This is often written in shorthand notation as KHP. How many grams of KHP are needed to exactly neutralize 21.0 mL of a 0.281 M potassium hydroxide solution ? grams KHC,H,O, Submit Answer Retry Entire Group 9 more group attempts remaining Potassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory to standardize strong base solutions. It has the unwieldy formula of KHCH.0. This is often written in shorthand notation as KHP. What volume of a 0.284 m potassium hydroxide solution is needed to exactly neutralize 1.62 grams of KHP? ANSWER: mL potassium hydroxide Submit Answer Retry Entire Group more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


