Question: Use the References to access important values if needed for this question. The elements tantalum (Ta) and boron (B) form two binary compounds with the
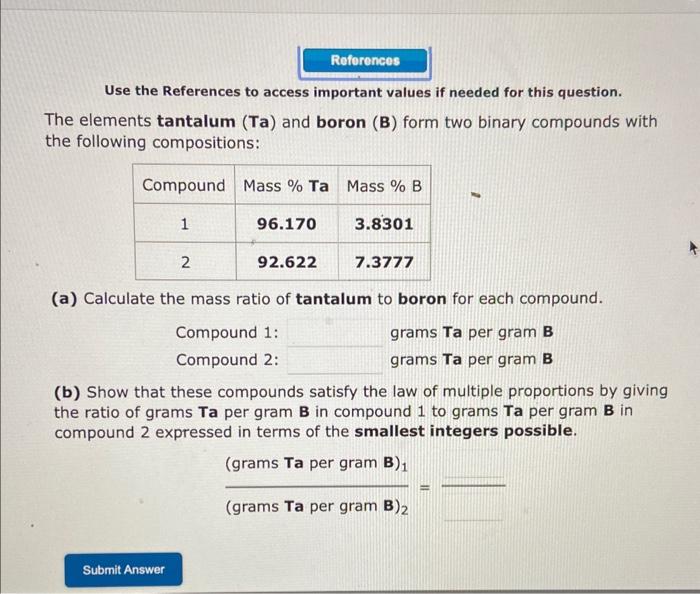
Use the References to access important values if needed for this question. The elements tantalum (Ta) and boron (B) form two binary compounds with the following compositions: (a) Calculate the mass ratio of tantalum to boron for each compound. Compound 1: grams Ta per gram B Compound 2: grams Ta per gram B (b) Show that these compounds satisfy the law of multiple proportions by giving the ratio of grams Ta per gram B in compound 1 to grams Ta per gram B in compound 2 expressed in terms of the smallest integers possible. ( grams Ta per gram B) 1 ( grams Ta per gram B) 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


