Question: Use the References to access important values if needed tor this a. When 81.823 and 71.94 are divided, the answer should be based on Enter
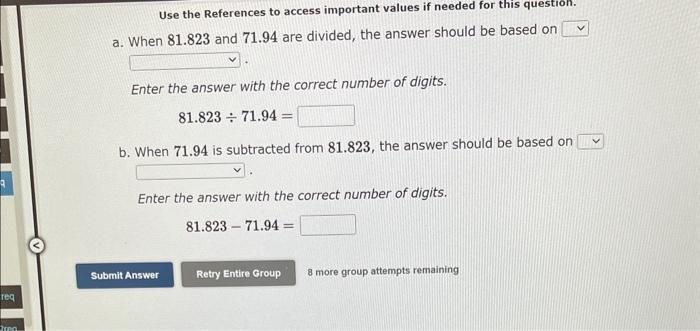
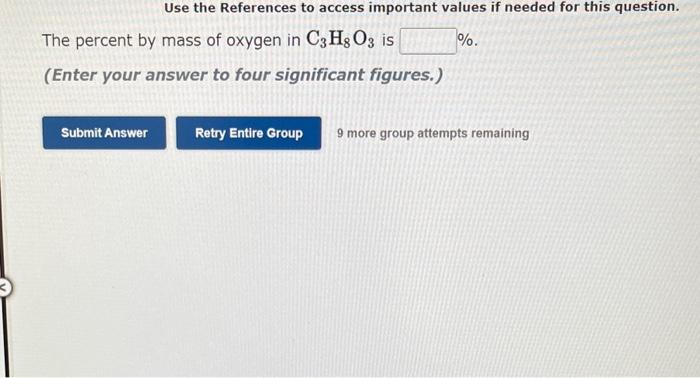
Use the References to access important values if needed tor this a. When 81.823 and 71.94 are divided, the answer should be based on Enter the answer with the correct number of digits. 81.82371.94= b. When 71.94 is subtracted from 81.823, the answer should be based on Enter the answer with the correct number of digits. 81.82371.94= 8 more group attempts remaining Use the References to access important values if needed for this question. The percent by mass of oxygen in C3H8O3 is % (Enter your answer to four significant figures.) 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


