Question: Use the specific heat interactive to calculate the specific heat of gold. First, determine the mass of water and the mass of gold. mwater 60
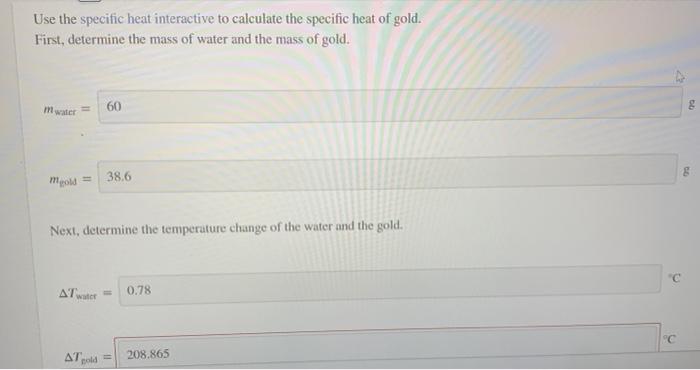
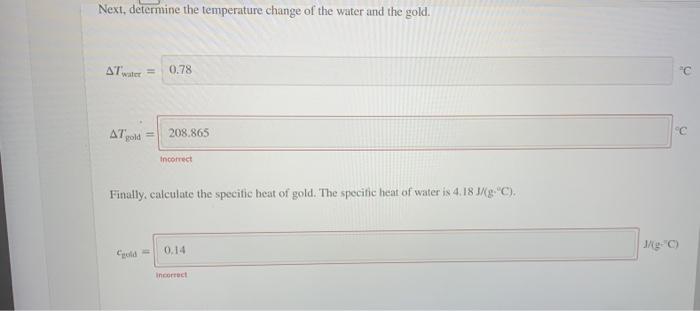
Use the specific heat interactive to calculate the specific heat of gold. First, determine the mass of water and the mass of gold. mwater 60 38.6 Next, determine the temperature change of the water and the gold AT 0.78 " old A 208.865 Next, determine the temperature change of the water and the gold, Atwater 0.78 "c AT gold 208.865 C Incorrect Finally, calculate the specitic heat of gold. The specific heat of water is 4.183/8 "C). 0.14 Me: incorrect Use the specific heat interactive to calculate the specific heat of gold. First, determine the mass of water and the mass of gold. mwater 60 38.6 Next, determine the temperature change of the water and the gold AT 0.78 " old A 208.865 Next, determine the temperature change of the water and the gold, Atwater 0.78 "c AT gold 208.865 C Incorrect Finally, calculate the specitic heat of gold. The specific heat of water is 4.183/8 "C). 0.14 Me: incorrect
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


