Question: Use the thermochemical equation: x e F 2 ( g ) + H 2 ( g ) 2 H F ( g ) + x
Use the thermochemical equation:
Together with the average bond energies given below, to calculate the average bond energy of the bond in
Bond Average Bond Energy
a
b
e
d
e
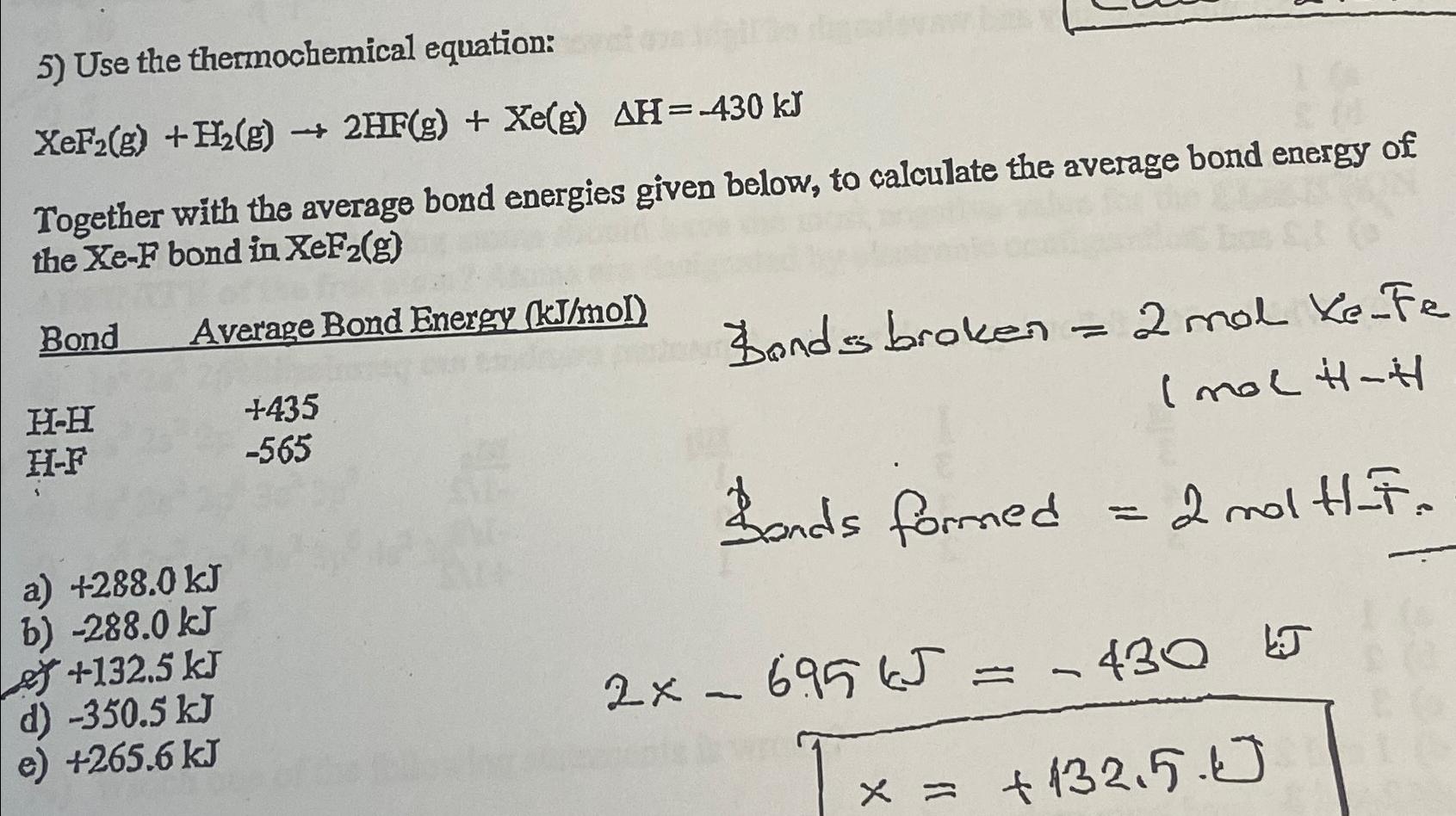
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


